Plant Hormones-Ethylene
In olden days, villagers, even now, used to accelerate the ripening process of banana, mango and other fruits, just before they were taken to market places. The method employed by them was simple. They used to keep the raw and unripened fruits in tightly closed earthen pots and fill the pot with smoke generated by burning cow-dung at the base and then seal it. After 12 to 16 hours of this treatment, the fruits would appear yellowish and just started for full ripening and they were ready for marketing (personal experience). Even today, villagers use this method without knowing why and how ripening is accelerated by the smoke generated by burning the cowdung. But plant physiologists discovered ethylene as the gas that induces and augments fruit ripening. This phenomenon and technology of fruit ripening was known to our village farmers many many centuries back. Even now renowned plant physiologists don't know this. All of us know cow dung produces atmospheric polluting gases such as methane and ethylene. For that matter animal fecal and fruits release more pollutants than all the cars (put together) that emit pollutants. Though scientific studies were initiated as early as 1900s, understanding the process of fruit ripening and identification of the causative factor was possible only in 1924. Since then, detailed studies have been made on ethylene and its effect on plants.

Structural formula C2H4; https://en.wikipedia.org/wikiEthylene; gcps.desire2learn.com
Many plant physiologists call ethylene as a plant hormone in gaseous state. But some do not agree with this view instead, they consider ethylene as a byproduct of reactions induced by other phytohormones and not an hormone perse. Two critical enzymes involved are SAM and ACC synthase. Both genes can be used for antisense technology for prolonging commercial fruit ripening, which is helpful for farmers for their products will have longer shelf life and this protectiveness has no deleterious effect on consumers. Now the release of transgenic savor flavor tomatoes in American market; it is difficult identify which is transgenic product which is not.
Ethylene is produced in response to exogenous stimuli as shown in the above diagram. In any of the plant developmental processes plant hormones interact with one another.

http://en.wikipedia.org/
Ethylene is produced essentially from all parts of higher plants, including leaves, stems, roots, flowers, fruits, tubers and seeds. Ethylene production is regulated by a variety of developmental and environmental factors. During the life of the plant, ethylene production is induced during certain stages of growth such as germination, ripening of fruits, abscission of leaves, and senescence of flowers. Ethylene production can also be induced by a variety of external aspects such as mechanical wounding, environmental stresses, and certain chemicals including auxin and other regulators.
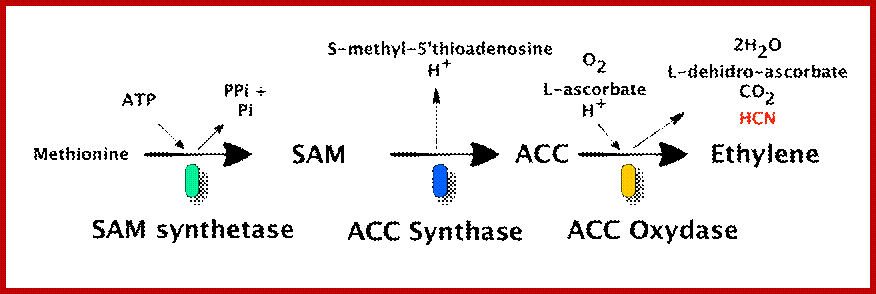
Ethylene is biosynthesized from an amino acid methionine to S-Adenosyl-L-methionine (SAM, also called Adomet) by the enzyme Met Adenosyl transferase. SAM is then converted to 1-aminocyclopropane-1-carboxylic acid (ACC) by the enzyme ACC synthase (ACS). The activity of ACS determines the rate of ethylene production, therefore regulation of this enzyme is the key for the ethylene biosynthesis. The final step requires oxygen and involves the action of the enzyme ACC-oxidase (ACO), formerly known as the ethylene forming enzyme (EFE). Ethylene biosynthesis can be induced by endogenous or exogenous ethylene. ACC synthesis increases with high levels of auxins, especially Indole Acetic Acid (IAA) and cytokinins.
The Yang cycle is shown. Plants use this pathway to synthesize ethylene. The picture has been generated using the following sources: Buchanan BB, Gruissem W, Jones RL (2000). http://en.wikipedia.org/

A web among plant regulators; www.onezer.com

Different Plant Hormones Regulate Similar Processes through Largely Nonoverlapping Transcriptional Responses; Jennifer L. Nemhauser, Fangxin Hong, Joanne Chory ; www.cell.com
The Brookhaven Plant Imaging Program uses non-invasive imaging of molecules tagged with short-lived radioisotopes including carbon-11 (half-life: 20.4 m), nitrogen-13 (half-life: 10 m) and fluorine-18 (half-life 110 m) to contribute directly to the DOE-OBER need for, “Fundamental research on microbes and plants to understand the genetic and biochemical mechanisms that control growth, development, and metabolism, provid(ing) knowledge needed … to develop new bioenergy crops and improved biofuel production processes that are cost effective and sustainable.”


Fastest growing plants are Bamboos not Sorghum; https://asiagardens.es

Bamboos; https://en.wikipedia.org

Plant imaging and Biochemistry; Hormonal signaling;https://www.bnl.gov/
In plants, hormonal signaling contributes substantially to growth and development, and to the regulation of physiological and biochemical traits relevant to bioenergy, including tissue architecture and stress resistance. Hormones, such as auxin, play major roles in all aspects of the development of form (morphology), physiology, and biochemistry. By understanding the mechanisms underlying plant signaling from hormone homeostasis to changes in gene expression, plant function and growth, we can potentially manipulate complex suites of traits, such as those conferring stress resistances.
Ethylene Pathway; SAM pathway; http://2013.igem.org/Team:UNITN-Trento
This 2-Oxogluterate Oxygenase/decarboxylase enzyme pathway found in some bacteria.

http://2013.igem.org/Team:UNITN-Trento
We all know Cyanide can
kill persons with in 10 minutes at very low concentration less than 300ppm. Fruit
renders hydrogen cyanide harmless thanks to the β-cyanoalanine synthase
enzyme, which catalyzes the synthesis of β-cyanoalanine from cysteine and
hydrogen cyanide. The issue is that the HCN removing reaction produces hydrogen
sulfide, which is also toxic and flammable! We weren't able to find a
biological way to remove the resulting hydrogen sulfide, so we searched for an
alternative ethylene synthesis pathways.
The plant pathway would have been a convenient way to produce ethylene. The
pathway contains SAM synthetase, an enzyme that our team was planning to
exploit for methyl salicylate production. Nevertheless, we continued looking
for alternatives. Plants have the ability to get rid of this hazardous
product. Fruits to generate and contain cyanide, but fruits get rid of it.
Distribution:
Ethylene is found in almost all parts of the plant body. But it is found in greater amounts particularly in old and yellowing leaves and ripening fruits. This compound being a gaseous substance diffuses through the intercellular spaces easily and rapidly reaches different regions of the plant body.
Biosynthesis:
Ethylene consists of two CH2 groups held by a common double bond H2C=CH2. The synthesis of ethylene is greatly enhanced by higher concentrations of auxin. Even Gibberellins and cytokinins induce the synthesis of ethylene indirectly. The precursor for ethylene was once believed to be methionine.
But recent investigations, using radioactive isotopes have shown that the precursor for ethylene is 1-amino cyclopropane carboxylic acid and not methionine. ACC acts as the direct and immediate precursor. In fact, higher concentration of auxin induces the synthesis of a group of ethylene synthetase enzymes. These enzymes require FMN, H2O and Cu2+ as the cofactors for their activity. The auxin induced enzymatic activity can be inhibited by actinomycin D and CHI, which suggests that ethylene synthases are inducible enzymes. The site of synthesis of ethylene has been suspected to be chloroplasts and its release is believed to be regulated by phytochromes.


Ethylene oxidation to CO2; www.skolaplnapohody.cz

Ethylene Biosynthesis in Arabinose thaliana; QIAGEN - GeneGlobe Pathways - Ethylene Biosynthesis in A. thaliana; www.qiagen.com
Apart from Auxins, many factors like wounding, aging, irritation, light, cold temperature and drought can also induce ethylene synthesis. Most of the above mentioned are stress factors; even ABA is known to induce ethylene production.
Effects:
Abscission: Onset of winter, cold treatment, drought and such conditions induce the formation of abscission layer in the stalks of leaves, flowers and fruits. Eventually the said structure separates by death of cells from the plant body and withers; it is also called in Greek language as Apoptosis. The structure and the development of abscission layer has been explained in the chapter ‘Auxin’. To put it in a nut shell, ethylene induces differential gene expression in the region, where the abscission zone develops. As a result, pectinase and cellulase enzymes produced and the same act upon the cell wall and degrade the same. Thus the abscission layer becomes the weak point and the leaves, fruits, etc., fall down by their sheer weight.

Ethylene causes abscission; www.plantphys.info
Fruit Ripening: Once the fruit reaches a particular stage of development, the raw, hard, green colored fruits undergo transformation to produce matured, soft, sweeter and yellow/red/orange/coloured fruits. The repining process requires period of time ranging from 24 hours to a week or so. And ethylene is known to initiate this phenomenon. Once the ripening is on, there is way to stop it.
With the maturity of fruits, the synthesis of ethylene is induced and ethylene induces more ethylene production. During early part of ripening process, ethylene initiates a cascade of events, which follow one after another and end up in a crescendo of biochemical reactions or what is called climacteric state at which all the biochemical reactions are at their maximum efficiency.
Ethylene to be effective in its action requires a copper containing metallo protein. Strangely, CO2 is known to bind to the same site of the protein at which ethylene binds and thus it competitively inhibits ethylene action. The active ethylene in its complex form first activates respiratory process by which reserve food materials and organic acids if found, are subjected to oxidative and decarboxylation reactions. The increase in respiratory activity is unusually cyanide insensitive, which means that the electron transport chain used in this process appears to be different from usual mitochondrial electron transport chain. Studies in this regard have revealed that the electron transport chain in this process branches of from Cyt.C and bypasses the cyt.a3 oxidase enzyme which is actually the site of cyanide inhibition. Most of the respiratory and other metabolic pathways that are stimulated by ethylene lead to the formation of more and more of sucrose and organic acids.
Fruit ripening can be climacteric or non-climacteric; in climacteric fruit generate large amount of ethylene as ripening proceeds. In the case of non-climacteric fruit ripen with little ethylene production; Examples- climacteric – Apple, Apricot, Avocado, Banana, Blueberry, Fig, Kiwi fruit, Mango, and papaya. Non-climacteric- Cherry, cucumber, Grape, lemons ,Pineapple, Strawberry, Sweet orange and Tamarillo (tree tomato).

Effect of Ethylene on Banana fruit; http://samohigarden.blogspot.in/
Banana ripening, I observed when I was very young; they use to bring fresh green Banana a day before putting into market we call it in villagers as “SANTHE”; put them is large earthen pot which has an opening at the bottom just above the base. Close the top and seal it with cow dung. Then they put dried cow dung with little raw cow dung and fire the cow dung plaques; as the raw cow dung gets fired they used to close it by a plate and seal it. Next day morning when you open the top one finds lovely yellow coloured banana fruits. I realized it when I was studying Masters course and teaching B,Sc students in the National college, Bangalore. The raw cow dung as it is heated it produces ethylene and the ethylene produced ripens the raw banana fruits within 10-12 hrs. Our Indian village agriculturist did not know the effect of Ethylene that is generated by the cow dung.

www.plantsinaction.science.uq.edu.au

Ethylene; www.plantphys.info
As the respiratory activity is reaching its climax ethylene simultaneously affects the membrane permeability and also activates a set of genes resulting in the synthesis of specific mRNAs. On translation of these mRNAs, specific proteins such as pectinase and cellulases are produced. The enzymes then act on the middle wall and primary walls to loosen up the cells, thus render the hard fruit into soft fruit. Ethylene induced fruit ripening can be effectively inhibited by actinomycin and CHI, which suggests that ethylene induces differential gene expression.

Ethylene induces differential gene expression, which can demonstrated by using ActinomycinD and CHI; www.imgarcade.com

Changes during ripening; www.plantphys.info

www.plantphys.info

www.plantphys.info
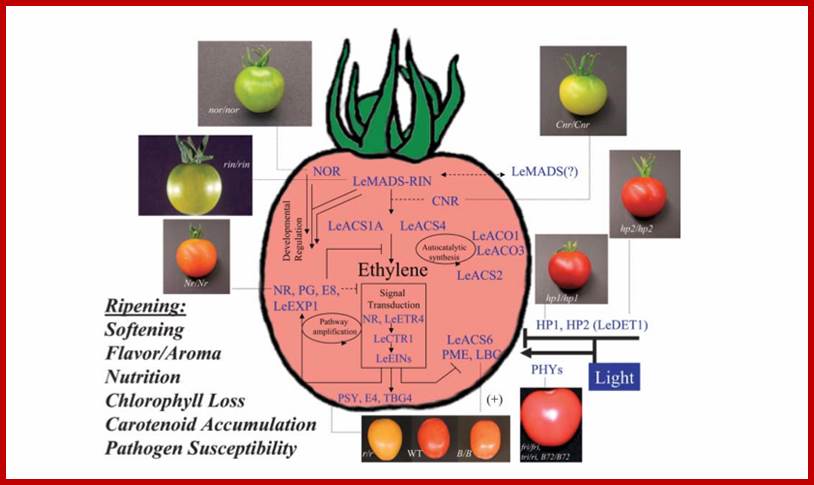
1. The rate limiting step in ethylene synthesis is the conversion of S-adenosyl methionine to 1-amino-cyclopropane-1carboxylic acid (ACC) via ACS synthases. ACS genes LeACS1A, LeACS4, LeACS2 and LeACS6 are under developmental control and are responsible for the initiation of ripening ethylene. Both are induced at the onset of ripening, and this induction is impaired by mutation at the ripening-inhibitor (rin) locus (Barry et al., 2000). Fruit homozygous for the rin mutation fail to exhibit ripening associated with ethylene production. Therefore, these genes would be good genes to look into. http://gcat.davidson.edu/
Banana is a climacteric fruit can be ripened by ethylene treatment. Process starts with change in fruit skin color, hardness to softness, and during climacteric stage ethylene is produced, chloroplast become chromoplasts, cellulose gets degraded, starch is converted to sugars, all leads to abscission layer formation at the base of the fruit stalk.


http://jxb.oxfordjournals.org/
Transcriptional control of fleshy fruit development and ripening:
Fleshy fruit types and their morphology. (A) Pericarp layers characteristic of capsular fruits (left) and fleshy fruits (right), fromPabón-Mora and Litt (2011). Comparative anatomical and developmental analysis of dry and fleshy fruits of Solanaceae.American Journal of Botany 98, 1415–1436; with permission. (B) Floral tissue origin of fruit. Ovary and ovary-derived tissue are represented in purple, and accessory tissues in yellow. The pericarp, which originates from the ovary wall, can be divided into several layers: exocarp (exo.), mesocarp (meso.), and endocarp (endo.). Seeds are represented in brown. Completed fromIreland et al. (2013). Apple SEPALLATA1/2-like genes control fruit flesh development and ripening. The Plant Journal 73,1044–1056. (C) A time series of tomato fruit development and ripening from flower to the red ripe stage.; journal of Experimental Botany; http://jxb.oxfordjournals.org/
Ethylene also affects the membrane stability and permeability. As a result, the pigments found in the tonoplast leak out and most of the membrane structures get disturbed. Furthermore, ethylene induces the degradation of chlorophyll by chlorophyllase which is again a product of gene expression. Simultaneously some anthocyanins are also synthesized which develop attractive coloration to the skin and the flesh of the fruit. Thus ethylene ultimately makes the fruit into a softer, sweeter and colorful commodity.
Effect on apical dominance;
Plants which show conical growth, ex., conifers, is known to have a strong apical dominance effect on the lateral buds. This has been attributed to strong influence of IAA present in the apical meristems found in the main axis. Recent investigations, it has been found that IAA induced apical dominance is more due to ethylene production than to auxin itself. Apical meristems of the main axis synthesize auxin and the same is translocated downwards. At the same time, some amount of IAA produced in very young leaves is also translocated towards the stem and more of auxin gets accumulated in the nodal regions. As higher concentration of IAA stimulates the synthesis of ethylene, which on synthesis, diffuses into lateral buds inhibits the growth. So the apical dominance is actually enforced by IAA through its second messenger i.e. ethylene. But the apical dominance can be overcome by the application of cytokinin, which removes the mitotic block imposed by ethylene and activates cell division, so the lateral buds grow into branches.
Effect on Geotropic Movements:
Geotropic responses are explained as due to the sensitivity of stem tip and root tip to different concentrations of auxins. But ethylene, a product induced by higher concentration of auxin brings about a reverse of geotropic curvatures called ageotropic effects, where roots instead of growing downwards into the soil curl upwards. In fact, ethylene treated roots loose their sense of directional growth. Sometimes, the effect of ethylene will be similar to the effects of morphactins. In addition, the other effects induced by ethylene, such as stunting, stem enlargement and prostrate habit by an impaired response to gravity are called ‘Triple response’ to ethylene.
Ethereal: In recent years, ethylene derivatives are sold in the market as ethereal or ethephon, a patented product. This compound is nothing but 2 chloro ethyl phosphonic acid. When these compounds are applied to plants in solution form, they release ethylene which in turn brings about its effects. Application value of this compound in agriculture, pomiculture is very well exploited during harvesting cotton balls and other fruit products. Application of ethereal induces not only ripening in most of the fruits irrespective of the age and degree of ripening, it also induces the abscission layer formation uniformly in stalks, which greatly facilitates harvesting either by mechanical means or manually at any given time.
Ethylene signal transduction pathways:
Ethylene though exists in gaseous form, it diffuses across cells, but when it enters a cell it binds to specific receptor. The signal transduction process are more like RTK but for they have histidine kinase activity. Very often in some cases it looks like an RTK pathway. The ultimate effects are different depending upon the organ on which it works and the time at which it works. Below only the self explanatory diagrams are give for your reference and looking for more information.


Ethylene is perceived by two component system, similar to bacterial system. The receptors contain Hk domain and R-domain; they activate MAP kinases which transmit through EIN2 membrane protein resulting in transcriptional cascade. Ethylene induced gene expression through cascade of kinase activity; http://www.mindfully.org/
Model for ethylene perception and signal transduction pathway:
Several theories on ethylene signal perception and transduction have been proposed to explain the mechanisms by which ethylene receptors could promote signal transduction through a cascade involving several components (Zarembinski and Theologis, 1994; Ecker, 1995; Bleecker and Kende, 2000).
The model recently proposed by Bleecker and Kende (2000) and subsequently reviewed by Giovannoni (2004) and Stepanova and Alonso (2005) places the components of the ethylene signal transduction pathway in a linear array and defends the theory that ethylene negatively regulates the joint binding of ETR1 and CTR1 to the receptor, resulting in de-repression of response pathways. The order of the components in this hypothetical linear chain is based on the analysis of epistasis genes, gene expression studies and the study of biochemical interactions. In this model, ethylene negatively regulates the family of receptors associated with the endoplasmic reticulum membrane and which are related to the two-component catalytic bacterial receptor family. The histidine-kinase transmitter domains of members of this receptor family interact with the CTR1 Raf-like kinase regulator domain. This CTR1 receptor/complex negatively regulates a membrane protein (EIN2) which is related to a super-family of metal-transporters. The C-terminal cytoplasmic EIN2 domain signals positively downstream of the EIN3 transcription factor family located in the nucleus. A target for the EIN3 transcription factors is the ERF1 gene promoter, which is a member of a second family of transcription factors and is rapidly induced in response to ethylene and is capable of activating a set of responses to ethylene when expressed.

A model of the role of EIN5 in the ethylene signal transduction pathway. Ethylene (C2H4) is perceived by repressing the action of receptor complexes including ETR/ERS/EIN4 receptors, RTE1, and Raf-like protein kinase CTR1, which negatively regulates downstream signaling component EIN2. Upon ethylene treatment, EIN2 is derepressed and could thus transmit the signal into the nucleus to activate a number of transcription factors, including EIN3 and EIL1. EIN3 directly binds to the regulatory elements of target genes and induces the expression of yet other transcription factors (i.e., ERFs and EDFs) that would ultimately regulate a series of ethylene responses. In the absence of ethylene signal, a Skp1-Cullin1-F-box complex consisting of one of two F-box proteins, EBF1 and EBF2, targets EIN3 protein for degradation via an ubiquitin/proteasome pathway. Interestingly, EBF1/EBF2 gene expression is induced by ethylene in an EIN3-dependent manner, which forms a negative feedback regulation on the EIN3 function. EIN5, a 5′→3′ exoribonuclease, is involved in facilitating the turnover ofEBF1/EBF2 mRNA through a yet unknown mechanism. Therefore, EIN5 is proposed to antagonize the negative feedback regulation on EIN3 by promoting EBF1 and EBF2 mRNA decay, which consequently allows the accumulation of EIN3 protein to trigger the ethylene response. Red arrows and blue bars represent positive and negative regulations, respectively. The dotted lines represent regulatory steps in which a direct physical link between upstream and downstream components has yet to be demonstrated.; http://www.pnas.org
The gaseous phytohormone ethylene is a key regulator in plant growth and developmental process as well as biotic and abiotic stress response. This review focuses on the recent advances in the ethylene-signaling pathway in Arabidopsis, with particular emphasis on the latest information about the downstream events of the ethylene-response pathway. Notable new findings include identification of a specific regulator of the ethylene receptor ETR1, discovery of protein degradation and RNA turnover processes in modulating EIN3-dependent transcriptional regulation, demonstration of the involvement of auxin biosynthesis in ethylene-mediated inhibition of root growth, and determination of possible integration points between ethylene and other hormonal and environmental signals (gibberellin, jasmonic acid, light, and sugar) in various plant processes. The elucidation of the molecular mechanisms of the ethylene-signaling and ethylene- response pathway in Arabidopsis might provide a framework for understanding how other plant species sense and respond to ethylene.

Overview of ripening regulation in climacteric fruits. The contribution of systems profiling approaches (shown at the top) will help identify novel regulatory genes and elucidate the interplay between epigenomic remodeling and transcriptional regulation involved during the ripening process; http://www.frontiersin.org/
Fruit ripening is a highly coordinated developmental process that coincides with seed maturation. The ripening process is regulated by thousands of genes that control progressive softening and/or lignification of pericarp layers, accumulation of sugars, acids, pigments, and release of volatiles. Key to crop improvement is a deeper understanding of the processes underlying fruit ripening. In tomato, mutations blocking the transition to ripe fruits have provided insights into the role of ethylene and its associated molecular networks involved in the control of ripening. However, the role of other plant hormones is still poorly understood. In this review, we describe how plant hormones, transcription factors, and epigenetic changes are intimately related to provide a tight control of the ripening process. Recent findings from comparative genomics and system biology approaches are discussed.

The genes shown represent a fruit ripening control network regulated by transcription factors (MADS-RIN, CNR) necessary for production of the ripening hormone ethylene, the production of which is regulated by ACC synthase (ACS). Ethylene interacts with ethylene receptors (ETRs) to drive expression changes in output genes, including phytoene synthase (PSY), the rate-limiting step in carotenoid biosynthesis. Light, acting through phytochromes, controls fruit pigmentation through an ethylene-independent pathway. Paralogous gene pairs with different physiological roles (MADS1/RIN, PHYB1/PHYB2, ACS2/ACS6, ETR3/ETR4, PSY1/PSY2), were generated during the eudicot (γ, black circle) or the more recent Solanum (T, red circle) triplications. Complete dendrograms of the respective protein families are shown in; http://www.nature.com/
Tomato (Solanum lycopersicum) is a major crop plant and a model system for fruit development. Solanum is one of the largest angiosperm genera1 and includes annual and perennial plants from diverse habitats. Here we present a high-quality genome sequence of domesticated tomato, a draft sequence of its closest wild relative, Solanum pimpinellifolium2, and compare them to each other and to the potato genome (Solanum tuberosum). The two tomato genomes show only 0.6% nucleotide divergence and signs of recent admixture, but show more than 8% divergence from potato, with nine large and several smaller inversions. In contrast to Arabidopsis, but similar to soybean, tomato and potato small RNAs map predominantly to gene-rich chromosomal regions, including gene promoters. The Solanum lineage has experienced two consecutive genome triplications: one that is ancient and shared with rosids, and a more recent one. These triplications set the stage for the neo-functionalization of genes controlling fruit characteristics, such as color and fleshiness.

Ethylene responsive gene activation; ucce.ucdavis.edu

Ethylene signaling and gene expression; www.qiagen.com
Ethylene Signaling in Arabidopsis
The Hydrocarbon Ethylene (C2H4) is a Gaseous Plant hormone, which is involved in a multitude of Physiological and Developmental processes. Responses to Ethylene include Fruit Ripening, Leaf Senescence and Abscission, Promotion or Inhibition of Seed Germination, Flowering and Cell Elongation. Environmental Stresses, such as Chilling, Flooding, Wounding and Pathogen Attack increase Ethylene Synthesis and thereby control Gene Expression. A combination of genetic, biochemical, and molecular approaches is uncovering this remarkable signaling pathway in plants. Although the initial hunt for the major elements of the Ethylene pathway was performed in the model plant Arabidopsis thaliana, identification and functional analysis of the corresponding genes in other plant species uncovered a high degree of conservation of this Signaling Cascade in the Plant Kingdom. Molecular genetic studies on the plant Arabidopsis have established a largely Linear Signal Transduction Pathway for the response to Ethylene gas. The signaling components of the Ethylene pathway include five Ethylene Receptors (ETR1, ETR2, EIN4, ERS1 and ERS2), which resemble Bacterial Two-component regulators; the MAPKKK (Mitogen-Activated Protein Kinase Kinase Kinase)-like protein Ctr1(Serine/Threonine-Protein Kinase Ctr1); EIN2 (Ethylene Insensitive Protein-2), a member of the N-Ramp family of metal-transporters; and the EIN3 and ERF families of transcription factors

Ethylene signalling, Stress tolerance and fruit ripening ; Ethylene signaling activates Ethylene response factors that bind to GCC box of stress responsive genes. Farid Regad et al; gbf.inp-toulouse.fr
Ethylene acts on its subject by using two component system as its signaling pathway. In Arabidopsis eight two component systems have been identified; among them five are ethylene signal transducers. Ethylene receptors ar ETR1, ETR2, ERS1, ERS2, and EIN4 show similarities with respect to histidine kinases. Ethylene been in a gas, its receptors are located on ER Endoplasmic reticulum. The binding of E2H2 to its receptors triggers signal and activates constitutively by stimulating protein kinase called Constitutive Ethylene Response 1 (CTR1) and activate specific gene transcription through 26s proteasome SCF-typeE3 ligase mediated degradation of repressors; http://www.sakshieducation.com/

http://www.sakshieducation.com/

The effects of ethylene on plants have been recognized since the Nineteenth Century and it is widely known as the phytohormone responsible for fruit ripening and for its involvement in a number of plant growth and development processes. Elucidating the mechanisms involved in the ripening of climacteric fruit and the role that ethylene plays in this process have been central to fruit production and the improvement of fruit quality. The biochemistry, genetics and physiology of ripening has been extensively studied in economically important fruit crops and a considerable amount of information is available which ranges from the ethylene biosynthesis pathway to the mechanisms of perception, signaling and control of gene expression. However, there is still much to be discovered about these processes and the objective of this review is to present a brief historic account of how ethylene became the focus of fruit ripening research as well as the development and the state-of- art of these studies at both biochemical and genetic levels . With the exception of ethylene, the signaling pathways that regulate fruit ripening remain largely undefined. . www.scielo.br; http://gcat.davidson.edu/
Regulation of ethylene-induced transcription of defense genes;
Ethylene-induced gene expression has been studied in systems in which the biosynthesis of ethylene is stimulated during developmental process such as ripening of fruit, senescence of flower petals, or during pathogen infection. Functional analysis of the promoters of these genes revealed that the ethylene-responsive cis-elements of fruit ripening genes and senescence genes differed from that of defense genes whose expression is induced by ethylene in response to pathogen infection. The ethylene nd pathogen-responsive element identified as the TAAGAGCCGCC ‘GCC’ box (AGCCGCC) is commonly found in the promoter region of the ethylene-inducible defense genes. The ethylene responsive element binding factors that interact with the GCC box were demonstrated to be the transcription factors, which respond to extracellular signals to modulate GCC box-mediated gene expression positively or negatively. Ethylne responsve elements are GCC box (AGCCGCC) is commonly found in the promoter region of the ethylene-inducible defense genes. Ethylene induced Osmotin like protein in tobacco plants gas the same promoter elements as on finds in water melon and tomato fruits. Promoter elements in petals defense genes have response elements such as GCC box (AGccGCC). http://www.ncbi.nlm.nih.gov/; http://www.ncbi.nlm.nih.gov/