Plant Hormones-Gibberellins
Gibberellic acid was first discovered in Japan under unusual circumstances. Farmers in Japan, before the World War, found that their paddy crop plants were afflicted with a strange disease called Bakane. The diseased plants showed unusual growth where plants were very tall, weak and sterile. This disease affected 20-30% of the crop plants and the farmers were subjected to great loss.


Gibberellins are diterpenoid carboxylic acids, and come in many forms. This is GA4, with the carbon atoms numbered according to convention and as referred to in the text. The two new papers show that in both GA3 and GA4 the carboxylic group on C6 and the hydroxyl group on C3, which are essential for biological activity, promote binding to the receptors. A hydroxyl group on C13, which is present in GA3 but absent in GA4, neither promotes nor hinders binding, whereas a hydroxyl group on C2 seriously reduces binding affinity. Hydroxylation on C2 is an important mechanism in higher plants for deactivating GAs. www.nature.com


Hormone and Light induced signal transductional event- generals; http://www2.mcdaniel.edu/
Investigation into the cause of abnormal growth called Bakanae disease of rice plant, Hori, a Japanese plant pathologist discovered that this disease was due to a fungus called Gibberella fujikuroi, but it is now identified as Fussarium moniliforme. Later Sawada and Kurosowa found that the extracts of this fungus simulated what the fungus could cause on infection. Then a group of Japanese workers led by Yabuta and Sumuki obtained an active principle from the extracts of the causative fungus, and later Yabuta and Hayashi identified the active principle as Gibberellic acid, which is new popularly called as Gibberellins (GA). Western scientists did not know anything about these discoveries before the First World War. After the war, they came to know about GA. Then they searched for this substance in higher plants and found this substance in all known higher plants. In fact they succeeded in obtaining GA in pure crystalline form and also they established its chemical structure.

GA3

Bakanae disease on Rice plants- longer plants; http://www.dpi.nsw.gov.au/


Maryland mammoth; Left fig,Maryland mammoth plastids are used to for production of HIV-1 viral to get p24 antigen; Maryland Mammoth mutant of tobacco (right) compared to wild-type tobacco (left). Both plants were grown during summer in the greenhouse. (University of Wisconsin graduate students used for scale.) (Photo courtesy of R. Amasino.) Dwarf and GA treated tobacco plants; www.abc.net.au; http://www.europeanmedical.info/

GA induced plant height vs left dwarf; http://aob.oxfordjournals.org/
Distribution
Almost every kind of plant, including algae, ferns, fungi, bacteria, is known to contain GA. Quantitative analysis of various parts of the higher plants shows that young leaves, which are just unfurling, are rich in GA. Even germinating seedlings contain significant amount of GA. Further studies revealed that proplastids found in young and developing leaves act as the site of GA synthesis and it is of great interest to know that their synthesis and release from plastids is under the subtle control of phytochromes. Though GAs is synthesized in plastids, they are translocated to different regions of the plant body through sieve tubes by active translocation mechanism.

The diagram shows the concentrations and spatial distribution of different phyhormones in a plant body under normal conditions. www.ukessays.com
Structure and Classification of GA
Gibberellins are diterpenoid acids derived from tetra cyclic diterpenoid known as Kaurene. The basic carbon skeleton of GA is known as Gibbane ring. But today the term Gibbane is in use. However, the systematic nomenclature of GAs is based on Kaurene and Gibbane structures.
Modified solvent extraction methods and the use of sophisticated analytical tools like HPLC etc. have greatly helped plant biochemists in identifying different forms of GAs. So far, 52 to 57 different kinds of Gas have been identified. Some of the gibberellins are known to be conjugated with glucose as glucose esters.
Chemical analysis of different parts of the plant body for GAs shows that different kinds of GAS are located in different parts of the plant body; their content and kinds vary at different stages of development of the plant body. Two or more GAs are found in the same plant but in different organs. In addition, Gibberellins exist in a dynamic state, in the sense; they undergo rapid interconversions from one kind to another, which however depends upon the intrinsic or extrinsic factors. It is also known that some of the gibberellins which are active in one organ are found to be inactive in another organ and vice versa. The dynamic equilibrium between different kinds of GAs, their synthesis and their multifaceted effects in plants is really fascinating.
Biosynthesis:
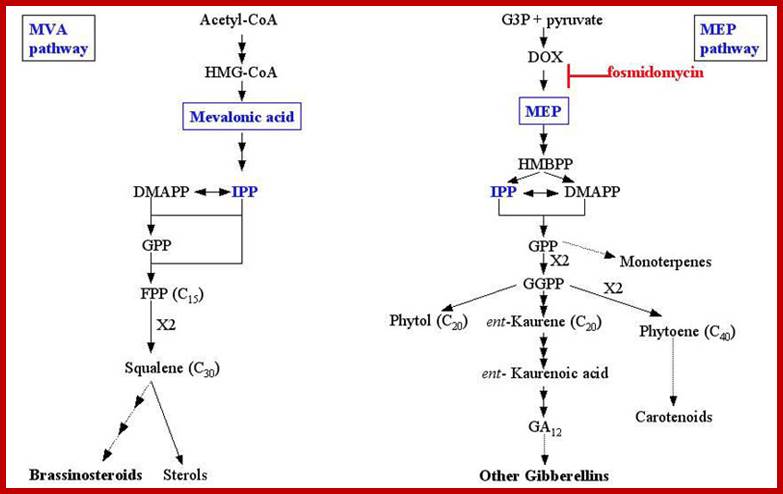
Biosynthesis of GA takes place in young plastids. Acetate is the precursor for the synthesis of all kinds of gibberellins.
In a series of multi-step reactions acetate is used to produce a diterpene compound called Kaurene. Specific enzymes responsible for each step have been more or less identified. The site of synthesis is proplastids or plastids or both. Plastids are also the site of fatty acid synthesis in plants. Still the work is going on to identify the number of GA forms- so for 126 GAs have been identified. GA interacts with other hormones such as AUXINs. Most active GAs are GA1, GA3, GA4 and GA7.
GA biosynthetic pathway:

Later steps in the GA pathway in pea shoots: Steps regulated by feedback, light, and/or auxin are indicated. GA1 is the bioactive GA; GA8 and GA29 are deactivation products. The le-1 mutation impairs the activation step, GA20 to GA1.www.5e.plantphys.net

GA biosynthesis Pathway in Plastids; Plant Cell Biology; www..pcb.org
Plant cell Biology; www.sciencearchive.org.au; http://5e.plantphys.net/

Gibberellins biosynthesis pathway; residing in 3 different cellular compartments (plastid, endoplasmic reticulum and cytoplasm). GGDP, geranylgeranyl diphosphate; ent-CDP,ent-copalyl diphosphate; CPS, ent-copalyl diphosphate synthase; KO, ent-kaurene oxidase; KAO, ent-kaurenoic acid oxidase. http://www.ncbi.nlm.nih.gov/
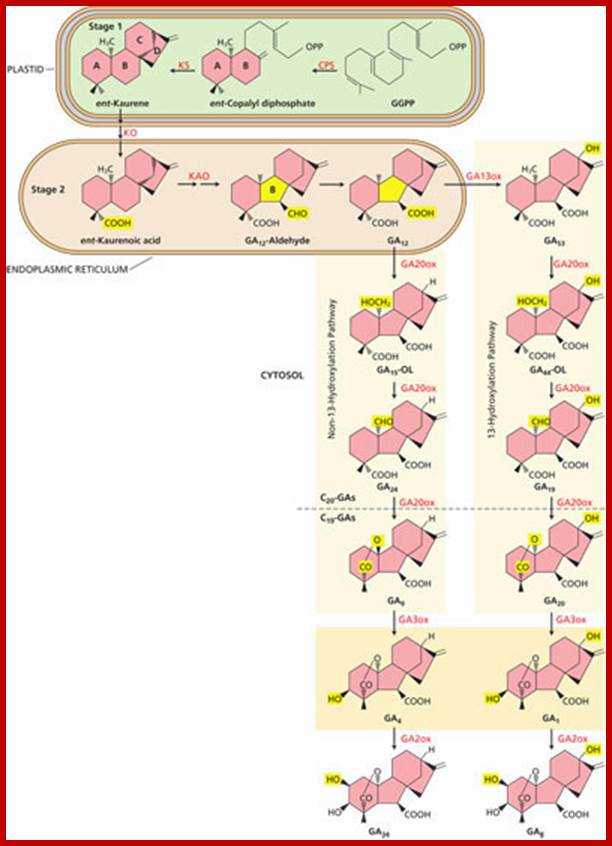
The three stages of GA biosynthesis. In stage
1, isopentenyl diphosphate is converted (not shown) to geranylgeranyl
diphosphate (GGPP), which is then converted to ent-kaurene
via ent-copalyl diphosphate in plastids. In stage
2, which takes place on the plastid envelope and endoplasmic reticulum, ent-kaurene
is converted to GA12. In many plants, GA12 is converted to GA53 by hydroxylation at C-13. In most
plants, the 13-hydroxylation pathway predominates, although in Arabidopsis and
some others, the non-13-OH-pathway is the main pathway. In stage 3, in the
cytosol GA12 or GA53 is converted, via parallel pathways, to
other GAs. This conversion proceeds with a series of oxidations at C-20,
resulting in the eventual loss of C-20 and the formation of C19-GAs.
In the non-13-hydroxylation pathway, this leads to the production of GA9.
GA9 is then oxidized
to the bioactive GA4 by
a 3β-hydroxylation reaction. In the 13-hydroxylation pathway, GA53 is sequentially oxidized at C-20,
leading to GA20, which is then 3β-hydroxylated to yield
bioactive GA1. Finally, hydroxylation at C-2 converts GA4 and GA1 to the inactive forms, GA34 and GA8, respectively. CPS
=ent-copalyl
diphosphate synthase; KS = ent-kaurene synthase; KO = ent-kaurene
oxidase; KAO = ent-kaurenoic acid
oxidase; GA13ox = GA 13-oxidase; GA20ox = GA 20-oxidase; GA3ox = GA 3-oxidase;
GA2ox = GA 2-oxidase; OL = open lactone.
(Click image to enlarge.); http://5e.plantphys.net/


http://www.nature.com/
Gibberellins (GAs) are considered as potentially important regulators of cell elongation and expansion in plants. Carrot undergoes significant alteration in organ size during its growth and development. However, the molecular mechanisms underlying gibberellin accumulation and perception during carrot growth and development remains unclear. In this study, five stages of carrot growth and development were investigated using morphological and anatomical structural techniques. Gibberellin levels in leaf, petiole, and taproot tissues were also investigated for all five stages. Gibberellin levels in the roots initially increased and then decreased, but these levels were lower than those in the petioles and leaves. Genes involved in gibberellin biosynthesis and signaling were identified from the carrot DB, and their expression was analyzed. All of the genes were evidently responsive to carrot growth and development, and some of them showed tissue-specific expression. The results suggested that gibberellin level may play a vital role in carrot elongation and expansion. The relative transcription levels of gibberellin pathway-related genes may be the main cause of the different bioactive GAs levels, thus exerting influences on gibberellin perception and signals. Carrot growth and development may be regulated by modification of the genes involved in gibberellin biosynthesis, catabolism, and perception.;Guang-Long Wang et al, http://www.nature.com/
During GA synthesis kaurene thus synthesized is converted to Gibberellic acid, which is then subjected to hydroxylation, methylation or glycosylation to produce different forms of Gibberellins. The remarkable feature of the biosynthetic pathway of GAs is that the same pathway is also used by plastids to synthesize certain plant pigments, Abscisic acid and some sterols. The needed enzymes for the biosynthesis of the above said compounds are regulated by phytochromes and other factors. The plastogenome and nuclear genome play a significant role in regulating the biosynthesis of Gibberellins and other mentioned compounds; how plastogenome contributes to the synthesis and its regulation is not known. Embryo looks like the site of biosynthesis of GAs.
Effects of GA on Plants;
GA is growth stimulating hormone; it stimulates growth in stem elongation, effects seed germination, effects meristem to shoot growth, vegetative to flowering, vegetative to flowering, sex expression and grain development, seed germination and interacts with environmental factors. It is not clear how GA moves in plant effects at gene level. Often one can observe counteracting effects of GA and ABA and supportive action of Auxin and GAs.
GA stimulates Auxin synthesis and Auxin stimulates cell elongation; and cell division and elongation; GA dos not induce turgor pressure or osmotic pressure; GA stimulates both cell elongation and cell division; does not increase osmotic uptake, no proton extrusion.
In GA deficient mutants, pollen does not develop and sepals, petals and pistils remain underdeveloped leading to premature abortion of the flower. Stamen and /or flower receptacles are found as 2 rich sites for synthesis of GAs.
Effect on General Metabolism:

Plant hormonal interaction during dormancy release and germination; http://www.frontiersin.org/

Hormonal interaction during seed dormancy and germination; http://www.seedbiology.de/
Gibberellins, as growth promoting hormones, accelerate the rate of cellular metabolic pathways such as respiration, protein synthesis etc. During the germination of cereal grains, GA is known to promote mobilizing the food materials for the growing embryo. It plays a significant role in metabolizing lipids to glucose and then to sucrose through gluconeogenesis process. The promotive effect of GA on nitrogen metabolism and HMP pathway is very significant. GA is also known for its effect on membrane transformation through rapid turnover of lipids. Interestingly, GA also favor C3 pathway in C3 plants, but the same hormone adversely affects Hatch and Slack pathway in C4 plants. Thus GA exerts a wider influence on general metabolic pathways.

http://www.frontiersin.org/
1. Effect on Transcription and Translation:
The metabolic effects exerted by Gibberellins show variations and it depends upon the structure, species and developmental stage and tissue of the plant body that receives it. The presence GA binding proteins have been identified from various sources like Pisum epicotyls, Phaseolus etc. Mol. Wt. of such proteins has been determined as 6.0 x 10^4 to 5.0 x 10^5 Daltons. GAs are also known to elicit specific responses through receptor proteins. Most of the times GA acts through receptors which linked to a trimeric G proteins. In some it acts without it?.
In cucumber hypocotyls, GA3 promotes the replication of DNA in plastids; thereby it exhibits its specific effect on biogenesis of chloroplasts. Gibberellins are also known to stimulate transcriptional activity of DNA templates through the binding of GA to AMP rich regions of DNA. In some cases, GA’ effect on transcriptional activity has been attributed to the activation of RNA polymerase through their receptor proteins, where the synthesis of all species of RNAs is enhanced.
The most specific effect of GA on transcription has been observed in the germinating barley grains. GA3 induces the mRNA synthesis for alfa amylase enzymes in aleurone cells. Probably this effect of GA is one of the best exemplified actions of phytohormones on differential gone expression. In spite of having voluminous data about GA’s effect on different plant species, nothing is clear how different GAs bring about different transcriptional activities leading to morphological expressions.
Gibberellins are also known to activate dormant mRNPs and the translating machinery. This view is supported by the fact that GA enhances polysome formation even in the absence of concomitant synthesis of mRNA. Synthesis of alfa amylase enzyme in barley aleurone layers, appearance of some new proteins during bolting and flowering are few examples to demonstrate the effect of Gibberellins on protein synthesis.
Effect on Genetic Dwarfism:
Genetic dwarfism, in many plants including garden peas and some species of French beans, is controlled by single genes. Such genetic dwarfs respond very favorably to GA treatment and in response to the hormone they grow as tall as normal tall varieties. The plant respond to GA by increasing internode growth by cell elongation.

www.5e.plantphys.net

The Effect of Gibberellins on Dwarf Plants Both of the dwarf tomato plants in this photograph were the same size when the one on the right was treated with gibberellins. http://www.78stepshealth.us/
However the phenotypic transformation of genetic dwarfs into tall plants is not heritable. Quantitative analysis of tall and dwarf peas and bean plants reveal the presence of high concentrations of GA in tall plants than is dwarf varieties, which suggests that dwarfism is due to a single gene mutation affecting one of the steps in GA biosynthesis. Cross breeding experiment do support this view.
The fascinating aspect of Gibberellin’s effect on genetic dwarfs in its specific action is on internodal growth. The internodes respond favorably than any other parts. The elongation of internodes is possible by two simultaneous processes i.e. one by meristematic divisions of intercalary meristems and the second is by the elongation of meristematic cells. The exact mechanism by which cell elongation is achieved is not clear, nonetheless, GA induced early growth of internodes can be inhibited by colchicine, but not by the inhibitors of transcription and translation. This indicates that the cytoskeleton structures like microtubules are involved in GA mediated growth similar to that of auxin induced cell elongation.
Dwarf rice plants also respond very well to GA treatment. GA induced internode elongation in rice plants is due to the activation of inter-calary meristems. After few cell divisions, the cell derivatives elongated 80-1000 times the original size. Elongation, in this case, is due to the excretion of protons across the cell walls and labializing the cell wall to be plastic. Meanwhile, intracellular turgidity also builds up due to the action of GA on plasma membranes. The combined effect of loosening the cell wall, the increased turgour pressure results in the cell elongation. It also effects cell division. GA induces cell elongation /stem elongation cell plasticity by inducing cell wall without acidity at apoplast. GA induced growth is faster than Auxins.
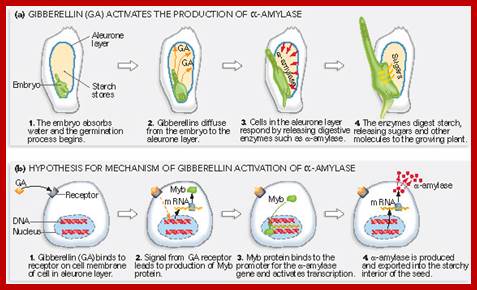
Effect on Aleurone Layers in Cereal Grains:
Cereal grains of Zea mays, sorghum, Hordium, Oryza etc have a distinct layer around the endosperm called aleurone layer, the cells of which are rich in protein granules. During germination, aleurone cells become active and with time, they secrete enzymes into endospermous tissue, where the reserve starch gets degraded to glucose and the same is utilized by the growing embryos.


www.plantphys.info; www.ib.bioninja.com.au

http://plantphys.info/plants_human
There is a definite relationship between the activity of aleurone cells and developing embryo. If the embryo is separated from the rest of the grain and incubated separately, aleurone cells fail to secrete any enzymes. On the other hand if the embryo is also incubated with the rest of the grain, after a period of time, aleurone cells start secreting alfa amylase. Instead of incubating embryo with the rest of the grain, if GA3 is added to the incubating medium, aleurone cells produce alfa amylase enzymes. Thus it is clear that during the early part of germination the young embryo produces Gibberellins which on reaching the aleurone layer activates the cells to produce the required enzymes like alfa amylase, protease, phosphoryl choline glyceride transferase and phosphoryl choline citidyl transferase. The last two mentioned enzymes are required in lecithin biosynthesis which activates membranes. Recently it has been reported that GA besides inducing the expression of alfa amylase inducing enzyme, it also triggers the expression of ribosomal RNA synthesis to a greater extent.

Growth promotion acts to enhance GA biosynthesis through upregulation of GA20ox and GA3ox; DELLA inhibitors which by signal transduction get degraded by 26S proteasome via interaction with GA receptors GID1 and SCF ubiquitin E3 ligase –mediated ubiquitination; http://jeb.biologists.org/

GA’s effect on aleurone cells; www.glycoforum.gr.jp

GA promoter elements of amylase gene; Hypothetical model for the control of Amy32b α-amylase gene expression in aleurone cells: A, Negative regulators (HvWRKY38, BPBF, HRT, and HvMCB1) bind to their corresponding cis-acting elements in the absence of GA. B, Positive regulators (RAMY, SAD, HvGAMYB, and HvMYBS3) bind to corresponding cis-acting elements in the presence of GA. Double lines between proteins indicate that their physical interactions have been detected by BiFC. The arrow denotes the transcription start site; X over the arrow means the transcription of Amy32b is off or at a very low level in the absence of GA; .http://www.plantphysiol.org www.pcb.org

Schemes of Constructs for αAmy3 and αAmy8 Promoter Analysis;
The αAmy3 SRC (–186 to –82 relative to the transcription start site) and αAmy8 SRC/GARC (–318 to –89) were fused upstream of a cauliflower mosaic virus (CaMV) 35S minimal promoter (35Smp)–alcohol dehydrogenase1 (Adh1) intron (In)–Luc–Nos 3′ chimeric gene. Relative positions of cis-acting elements, including GC, G, the GARE, and TA boxes, in promoters are indicated. http://www.plantcell.org/


Different mechanisms serve to inactivate DELLA repressors of the GA signaling pathway. (A) In the “standard” situation, GA-bound GID1 proteins interact with DELLA repressors and induce their ubiquitination and degradation via E3 ubiquitin ligases such as found in Arabidopsis SCFSLY1/SNZ or rice SCFGID2. (B) DELLA ubiquitination and degradation are defective in E3 ubiquitin ligase mutants such as sly1 or gid2. There, the GA-promoted GID1–DELLA interaction is sufficient to inactivate DELLAs and relieve DELLA-imposed growth restraints. (C) GID1 variants with a substitution of a conserved Proline (P) residue can interact with DELLAs in a GA-independent manner and promote GA signaling independent from the hormone. Arabidopsis. GID1b is a naturally occurring GID1 protein that has a histidine instead of the Proline (P → H). GID1 mutant analyses additionally revealed that P → A or P → S substitutions render GID1 GA-independent. http://journal.frontiersin.org/

http://plantphys.info/plant_physiology/gibberellin.
The synthesis of GA induced enzymes can be inhibited by actinomycin D or cycloheximides, which are the inhibitors of transcription and translation respectively. This suggests that GA preferentially activates the gene expression for the above said enzymes. Among differentially expressed genes, 475 and 925 genes were up regulated in grapes flowers and 316 to 604 genes are down regulated within 24 hrs of treatment.
Studies on GA induced molecular events clearly suggest than GA at very early stages activates preexisting mRNPs for alfa amylase but later the hormone activates specific genes in aleurone cells. However, the increase in the levels of transcription for amylase and protease reaches maximum at 6-7 hours after hormone treatment. The above results further supported by the fact, that when GA induced mRNAs from aleurone layer are translated in an vitro system, among the many proteins produced, the levels of alfa amylase and protease is found to be high. In actuality, the newly synthesized enzymes are loaded into membrane vesicles and the same is secreted out of the aleurone cells into endospermous tissue, where they bring about hydrolytic activity and thus mobilize the food materials for the developing embryos.
Endosperms cells are dead cells. In recent investigations it has been shown that GA not only induces the synthesis of mRNAs for alfa amylase. But ABA suppresses the GA induced mRNA for alfa amylase. However ABA does not prevent the synthesis of other RNAs.

Plant Cell Biol; www.images.1233.tw
GA signaling takes through trimeric G receptor protein. This leads to pathways, one-Ca+ dependent pathway and the other Ca+ independent pathway; not much is known about how GA induces signal transduction.
GA binds to surface receptor GPCR, which is associated with trimeric signal transduction components at inner surface of the PM bound to the receptor. Once the GPCR is activated signal transduction can lead to two pathways- one GPCR mediated and another direct.

Effect of G-alpha mutant in dwarf Rice affects G-protein receptor-transduction; Diagram summarizing the two proposed GA-signaling pathways in rice. (Model A) Gα works in high sensitive GA receptor system. There may be an alternative GA receptor system for low sensitivity to GA that does not involve Gα. High sensitive α-amylase induction and internode elongation by GA may be mediated mainly through the high sensitive reception system (red), whereas leaf sheath elongation may be through the low sensitive reception system (blue). (Model B) Gα works in a pathway that regulates the GA signaling. α-Amylase induction and internode elongation may be regulated mainly by this pathway to result in high sensitivities to GA (red), whereas leaf sheath elongation may be not regulated by this pathway to result in low sensitivity to GA (blue). The SLR protein works at the downstream site of these two pathways. http://www.pnas.org/
The Molecular Mechanism and Evolution of the GA–GID1–DELLA Signaling Module in Plants;
Fig. Below: GA-GID1-DELLARegulatory module highlighted in orange. Signals that promote bioactive GA accumulation are labelled blue, whereas signals that signals that reduce GA levels are highlighted in purple. DELLA interacts directly with purple regulatory proteins PIFs, SCL3, ALC and Jazz, highlighted in green to mediate cross talk between GA and other signaling pathways; http:// cell.com
Mas7, a cationic amphiphilic tetradecapeptide that stimulates GDP/GTP exchange by heterotrimeric G proteins, specifically induced alpha-amylase gene expression and enzyme secretion in a very similar manner to GA1. Bioactive GAs are GA1,GA3, GA4 aqnd GA7
It is involved plant developental activities, surprisingly It can cause male sterility.


http://catherine-wwwmyblog.blogspot.in/
Mas7, a cationic amphiphilic tetradecapeptide that stimulates GDP/GTP exchange by heterotrimeric G proteins, specifically induced alpha-amylase gene expression and enzyme secretion in a very similar manner to GA1.
Heterotrimeric G proteins are associated with the cytoplasmic face of the plasma membrane of a variety of eukaryotic cells and transduce information from cell surface G protein–coupled receptors to effector proteins of signal transduction pathways
A wealth of evidence indicates a role for G proteins in the regulation of K1 influx channels of stomatal guard cells (Assmann, 1996).
Obtained evidences suggest a role for heterotrimeric G proteins in signal transduction leading to a-amylase gene expression in wild oat aleurone. In addition, we describe the cloning, sequencing, and express the cloned genes.
GAs (Gibberellins) are members of a large family of Diterpenoid compounds, which are essential for a number of processes, including Gene Expression in Cereal Aleurones, Seed Germination, Elongation, Growth, and Flowering. During the last four decades, Barley Aleurone has been a valuable system for studying GA regulation of gene expression. After germination, GAs are released from the Embryo into the Endosperm, triggering the expression of a number of genes encoding Hydrolytic enzymes in Aleurone cells. Many of these Hydrolytic enzymes, which include Alpha-Amylase, Proteases, and Cell Wall–degrading enzymes, are secreted and are responsible for digestion of the stored reserves in the starchy endosperm. The Signal transduction events leading from the Receptor to the coordination of the Complex events that make up and regulate the secretory activity of these cells are still poorly understood. A range of downstream-signaling components and events has been implicated in GA signaling in Barley Aleurone. These include the G-Alpha subunit of a Heterotrimeric G-Protein, a transient elevation in cGMP (Cyclic Guanine Monophosphate), Ca2+ (Calcium ion) dependent and Ca2+ independent events in the Cytoplasm, reversible protein phosphorylation, and several promoter cis-elements and transcription factors, including GAMyb (Gibberellin Myb) ; https://www.qiagen.com.
GA is perceived on the
surface of Barley Aleurone cells by an unidentified outward-facing
Plasmamembrane–associated GA Receptor. Binding activates, directly or
indirectly, Second messengers and G-proteins. This interaction stimulates a
Signal Transduction Cascade that involves the phosphorylation or
dephosphorylation of proteins on Serine, Threonine, or Tyrosine. Eventually,
the Signal reaches the nuclear DELLA Protein, Sln1. The DELLA proteins are highly conserved negative regulators of
GA signaling in Barley. These DELLA proteins are named after a conserved Amino
Acid motif near their N-termini. The DELLA proteins form a subfamily within a
family of putative transcriptional regulators known as GRAS. Sln1 acts as a repressor of GA responses, inhibiting the
transcription of the gene encoding the GAMyb activator of the Alpha-Amylase response. The GA signal alters
Sln1, resulting in
Proteasome-dependent Sln1 destabilization. The inactivation of Sln1 repressor allows the expression of GAMyb genes, as well as other genes, to proceed through transcription,
processing and translation. The lag time between Sln1 disappearance and the expression of GAMyb remains poorly
understood. The newly synthesized GAMyb proteins then enter the nucleus and binds to the promotor genes
for Alpha-Amylase and other hydrolysing enzymes.
Spy (Spindly) protein negatively regulates GA responses in Aleurone.
Two of the HSI (Hordeum Spy-Interacting) Proteins, HSImyb and HSINac inhibited the
GA up-regulation of Alpha-Amylase expression in Aleurone. Recent evidence suggests that the
GA regulation of GAMyb involves both
transcriptional and post-transcriptional control. A GAMyb binding protein, Kgm, has been identified as a repressor of transcriptional
activation of an Alpha-Amylase promoter by GAMyb. Kgm, a Mitogen-Activated
Protein–like Kinase, is expressed in Aleurone cells in the absence of GA. HRT is a repressor of Alpha-Amylase gene expression. This nucleus-localized Zinc-finger protein
binds to a 21-bp sequence containing the TAACAAA element, but at present there
is no evidence for GA control of its repressor function (Ref.3 & 4).
Along with GAMyb, other early GA
responses in Aleurone cells include increase in cytosolic Calcium, Calm(Calmodulin), and ER
(Endoplasmic Reticulum)-localized Ca2+-ATPase. GA, in presence of Calcium,
increased the level of Calm in Barley Aleurone layers by twofold. Calm binds to Ca2+ ions, and the resulting Ca2+–Calm Complex is capable of activating specific enzymes, such as
Ca2+–Calm-dependent Protein Kinases. GA stimulates the secretion of Alpha-Amylase and other Hydrolases via a Ca2+-dependent pathway, whereas GA
appears to stimulate expression of the Alpha-Amylase gene via a Calcium-independent pathway. cGMP is a candidate for
a Calcium-independent signaling intermediate involved in GA-induced gene
expression. cGMP play an intermediary role between Sln1 and early response genes. Increase in cGMP in response to GA
correlate closely with increase in GAMyb protein in Barley Aleurone cells. An inhibitor of Guanylyl
Cyclase, the enzyme that synthesizes cGMP from GTP, blocks GA-induced Alpha-Amylase production (Ref.4 & 5). Various GA response complexes have
been identified in the promoters of Alpha-Amylase genes. TAACAAA-like sequence motifs present in these GA response
complexes play a key role in mediating the GA activation of transcription. The
addition of the TAACAAA element to a minimal 35S promoter conferred GA
responsiveness, indicating that the element can act as a GA response element. Alpha-Amylase and other Hydrolases are synthesized on the RER (Rough
Endoplasmic Reticulum). Then they are secreted via the Golgi. The Secretory
Pathway requires GA stimulation via Calcium-Calm dependent Signal Transduction
Pathway. In conclusion, GA Signal Transduction seems to involve Calcium ions as
well as cGMP, but the detailed Signaling pathways have not been worked out. Alpha-Amylase secretion is regulated by a Calcium-dependent pathway, whereas Alpha-Amylase gene expression is regulated by a Calcium-independent pathway
(Ref.6).
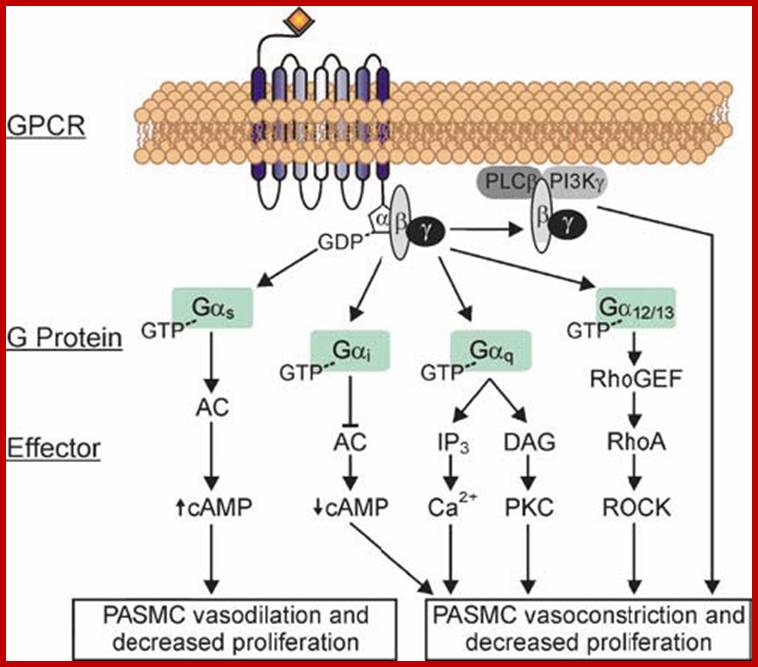
Receptor mediated signal Transduction and ;www.pvri.info
G-protein-coupled-receptor (GPCR)-mediated signaling in the pulmonary artery smooth muscle cell (PASMC) animal system. GPCR activation promotes the exchange of the G-protein-bound GDP for GTP, thereby inducing a conformational change and dissociation of Ga from the Gbg and thus initiating downstream signaling. The Gbg subunits can directly activate phosphatidylinositol 3-kinase (PI3K) and phospholipase Cb (PLCb). PLCb, activation (by Gbg and Ga q) promotes the hydrolysis of phosphatidylinositol 4,5-bisphosphate and yields the intracellular messengers 1,2-diacylglycerol (DAG) and inositol 1,4,5-trisphosphate. DAG remains membrane-bound and promotes the translocation of protein kinase C from the cytoplasm to the membrane and its subsequent activation. Ga12/13-dependent pathways (guanine nucleotide exchange factors for Rho activate the small G protein RhoA followed by Rho kinase) and Gai-dependent pathways (inhibition of adenylyl cyclase decreases cyclic AMP accumulation) lead to PASMC vasoconstriction and proliferation. In contrast Ga s stimulates the production of cyclic AMP via activation of adenylyl cyclase, which in turn decreases PASMC proliferation and enhances vasodilation.
The Ca+ independent pathway activates phosphorylation of DELLA proteins, which are repressors of GAMB gene (GA induced Amylase-beta). Phosphorylation of F-box proteins (SLN1 and SLR1) leads to ubiquitinated protein degradation. This facilitates the GA-GA receptor complex bind to GAMYB gene promoter and activates the transcription of the genes. Translated product GAMY beta protein, a transcriptional regulator protein, enter the nucleus and binds Amylase gene regulatory elements called GARE (GA response elements) and induce amylase gene expression. Transcripts on translation produce amylase proteins which are transferred into ER and loaded into vesicle and stored in cytoplasm. They are released in response to GA through what is called Ca+ dependent pathway. Note MYB is a family of genes there can be anywhere 80-108 in number; they are the most abundant transcription factors.

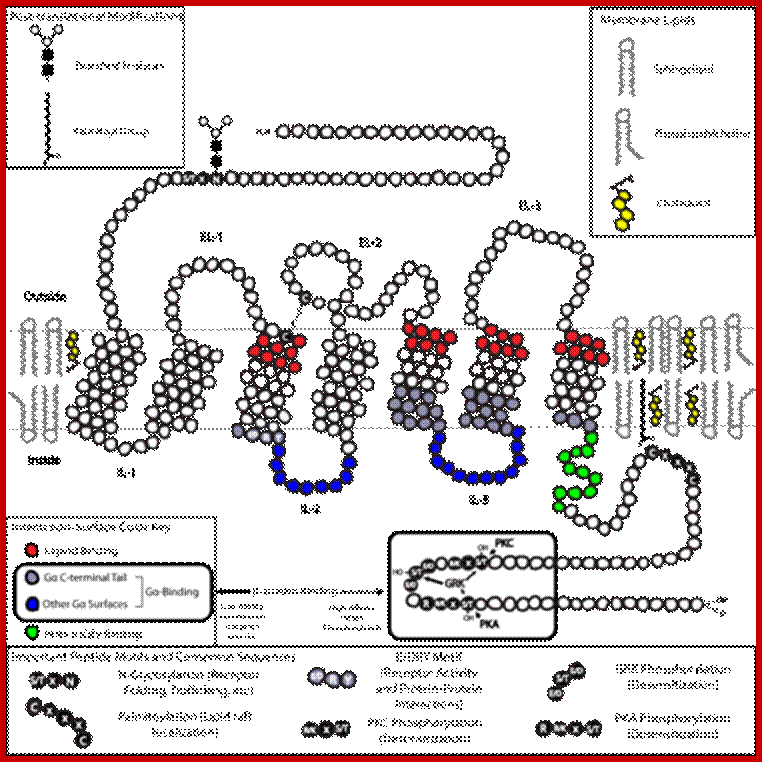
G protein–coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein–linked receptors(GPLR), constitute a large protein family of receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times.[2] Classification Scheme of GPCRs. Class A (Rhodopsin-like), Class B (Secretin-like),Class C (Glutamate Receptor-like), Others (Adhesion (33), Frizzled (11), Taste type-2 (25), unclassified .
Two-dimensional schematic of a generic GPCR set in a Lipid Raft. Click the image for higher resolution to see details regarding the locations of important structures; https://en.wikipedia.org;
GPCR; G protein–coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein–linked receptors(GPLR),
G-protein–coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and animals. The ligands that bind and activate these receptors include light-sensitive compounds, odors, pheromones, hormones, and neurotransmitters, and vary in size from small molecules to peptides to large proteins. G protein–coupled receptors are involved in many diseases, and are also the target of approximately 40% of all modern medicinal drugs.
The 2012 Nobel Prize in Chemistry was awarded to Brian Kobilka and Robert Lefkowitz for their work that was "crucial for understanding how G protein–coupled receptors function; GPCR have 5 families.
There are two principal signal transduction pathways involving the G protein–coupled receptors: the cAMP signal pathway and the phosphatidylinositol signal pathway; https://en.wikipedia.org.
The mastoparan analog Mas7. Mas7 is a cationic amphiphilic tetradecapeptide that stimulates GDP/ GTP exchange by heterotrimeric G proteins.
It is estimated that approximately 1000 different such receptors exist in mammals. The molecular mechanisms involved in GPCR function, particularly the molecular modes of receptor activation and G-protein recognition and activation, have therefore become the research focus of an ever increasing number of laboratories.
GPCR dimerises then induces Gprotein.
Gibberellin Signaling in Barley Aleurone Grain:
GAs (Gibberellins) are members of a large family of Diterpenoid compounds, which are essential for a number of processes, including Gene Expression in Cereal Aleurones, Seed Germination, Elongation, Growth, and Flowering. During the last four decades, Barley Aleurone has been a valuable system for studying GA regulation of gene expression. After germination, GAs are released from the Embryo into the Endosperm, triggering the expression of a number of genes encoding Hydrolytic enzymes in Aleurone cells. Many of these Hydrolytic enzymes, which include Alpha-Amylase, Proteases, and Cell Wall–degrading enzymes, are secreted and are responsible for digestion of the stored reserves in the starchy endosperm. The Signal transduction events leading from the Receptor to the coordination of the Complex events that make up and regulate the secretory activity of these cells are still poorly understood. A range of downstream-signaling components and events has been implicated in GA signaling in Barley Aleurone. These include the G-Alpha subunit of a Heterotrimeric G-Protein, a transient elevation in cGMP (Cyclic Guanine Monophosphate), Ca2+ (Calcium ion) dependent and Ca2+ independent events in the Cytoplasm, reversible protein phosphorylation, and several promoter cis-elements and transcription factors, including GAMyb (Gibberellin Myb) ; https://www.qiagen.com.
GA is perceived on the
surface of Barley Aleurone cells by an unidentified outward-facing
Plasmamembrane–associated GA Receptor. Binding activates, directly or
indirectly, Second messengers and G-proteins. This interaction stimulates a
Signal transduction Cascade that involves the phosphorylation or
dephosphorylation of proteins on Serine, Threonine, or Tyrosine. Eventually,
the Signal reaches the nuclear DELLA Protein, Sln1. The DELLA proteins are
highly conserved negative regulators of GA signaling in Barley. These DELLA
proteins are named after a conserved Amino Acid motif near their N-termini. The
DELLA proteins form a subfamily within a family of putative transcriptional
regulators known as GRAS.Sln1 acts as a repressor of GA responses, inhibiting the
transcription of the gene encoding the GAMyb activator of theAlpha-Amylase response. The GA signal alters Sln1, resulting in
Proteasome-dependent Sln1 destabilization. The inactivation of Sln1 repressor allows the expression of GAMyb genes, as well as other genes, to proceed through transcription,
processing and translation. The lag time between Sln1 disappearance and the expression of GAMyb remains poorly
understood. The newly synthesized GAMyb proteins then enter the nucleus and binds to the promoter for Alpha-Amylase gene and other hydrolyzing
enzymes. Spy (Spindly) protein negatively regulates GA responses in Aleurone.
Two of the HSI (Hordeum Spy-Interacting) Proteins, HSImyb and HSINac inhibited
the GA up-regulation ofAlpha-Amylase expression in Aleurone. Recent evidence suggests that the
GA regulation of GAMyb involves both transcriptional and post-transcriptional control.
A GAMyb binding protein, Kgm, has been identified as
a repressor of transcriptional activation of an Alpha-Amylase promoter by GAMyb. Kgm, a Mitogen-Activated
Protein–like Kinase, is expressed in Aleurone cells in the absence of GA. HRT is a repressor of Alpha-Amylase gene expression. This nucleus-localized Zinc-finger protein binds
to a 21-bp sequence containing the TAACAAA element, but at present there is no
evidence for GA control of its repressor function (Ref.3 & 4).
Along with GAMyb, other early GA
responses in Aleurone cells include increase in cytosolic Calcium, Calm(Calmodulin), and ER
(Endoplasmic Reticulum)-localized Ca2+-ATPase. GA, in presence of Calcium,
increased the level of Calm in Barley Aleurone layers by twofold. Calm binds to Ca2+ ions, and the resulting Ca2+–Calm Complex is capable of activating specific enzymes, such as
Ca2+–Calm-dependent Protein Kinases. GA stimulates the secretion of Alpha-Amylase and other Hydrolases via a Ca2+-dependent pathway, whereas GA
appears to stimulate expression of the Alpha-Amylase gene via a Calcium-independent pathway. cGMP is a candidate for
a Calcium-independent signaling intermediate involved in GA-induced gene
expression. cGMP play an intermediary role between Sln1 and early response genes. Increase in cGMP in response to GA
correlate closely with increase in GAMyb protein in Barley Aleurone cells. An inhibitor of Guanylyl
Cyclase, the enzyme that synthesizes cGMP from GTP, blocks GA-induced Alpha-Amylase production (Ref.4 & 5). Various GA response complexes have
been identified in the promoters of Alpha-Amylase genes. TAACAAA-like sequence motifs present in these GA response
complexes play a key role in mediating the GA activation of transcription. The
addition of the TAACAAA element to a minimal 35S promoter conferred GA
responsiveness, indicating that the element can act as a GA response element. Alpha-Amylase and other Hydrolases are synthesized on the RER (Rough
Endoplasmic Reticulum). Then they are secreted via the Golgi. The Secretory
Pathway requires GA stimulation via Calcium-Calm dependent Signal Transduction
Pathway. In conclusion, GA Signal Transduction seems to involve Calcium ions as
well as cGMP, but the detailed Signaling pathways have not been worked out. Alpha-Amylase secretion is regulated by a Calcium-dependent pathway, whereas Alpha-Amylase gene expression is regulated by a Calcium-independent pathway

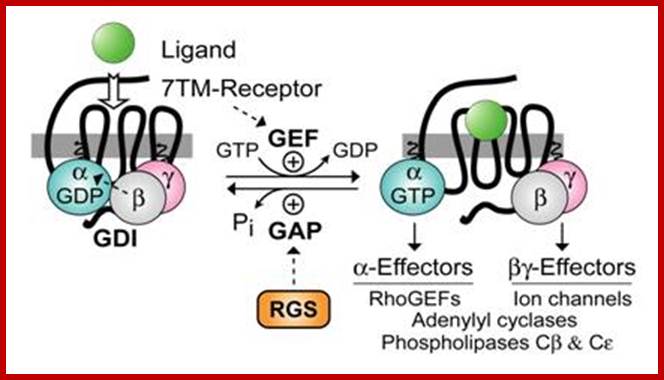
The heterotrimeric G‐protein cycle. This simplified model highlights elements of the pathway that are found in both metazoans and plants. The repertoire of each element in plants is greatly reduced compared with metazoans. GPCR, G‐protein‐coupled receptor; RGS, regulator of G‐protein signalling. Figure modified with permission from Assmann; http://embor.embopress.org.
G protein–coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein–linked receptors(GPLR), constitute a large protein family of receptors that sensemolecules outside the cell and activate inside signal transductionpathways and, ultimately, cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times.[2] Classification Scheme of GPCRs. Class A (Rhodopsin-like), Class B (Secretin-like),Class C (Glutamate Receptor-like), Others (Adhesion (33), Frizzled (11), Taste type-2 (25), unclassified (23)) . GPCR; G protein–coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein–linked receptors(GPLR), [10] https://en.wikipedia.org;

http://labs.bio.unc.edu/http://labs.blabs

Model for signal transduction by activation/inactivation of heterotrimeric G proteins through GPCR; The subunits of heterotrimeric G proteins (Gα and Gβγ) in their inactivated state are associated with each other. In inactivation state the GDP is bound to Gα (Gα-GDP). In signal transduction, first the GPCR gets activated by changing its conformation which resulted from binding of agonist/ligands to the extracellular region of GPCR. This activated GPCR further activate the inactive G protein to active G protein complex by dissociating the Gα from Gβγ. In active state the GTP is bound to Gα (Gα-GTP). Now free Gα and Gβγ have their own effectors (E1 and E2, respectively) to further transmit the signals and initiate unique intracellular signaling responses. Later, after the signal transduction, the Gα-GTPase activity hydrolyze the bound GTP (Gα-GTP) to GDP and Pi and inactivate the G protein complex by re-associating the Gα with Gβγ. In this state again GDP is bound to Gα (Gα-GDP) in the G protein complex. in this way the activation and inactivation cycle is completed.
Functional analysis of a barley high-pI alpha-amylase gene promoter has identified a gibberellin (GA) response complex in the region between -174 and -108. The sequence of the central element, TAACAAA, is very similar to the c-Myb and v-Myb consensus binding site. An additional element CCTTTT, which with the O2S forms part of a canonical "endosperm box," is important in modulating the absolute level of expression of the Amy32b promoter, as is another separate, highly conserved element TATCCATGCAGTG, this hormone-responsive region is located between -174 and -108. The conserved motifs (-169 pyrimidine box), -143TAACAAA box, and -124TATCCAC box and nonconserved intervening sequences within the region between -174 and -108. Our results showed that both the TAACAAA and TATCCAC boxes play an important role in GA-regulated expression. We propose that the TAACAAA box is a gibberellin response element, that the TATCCAC box acts cooperatively with the TAACAAA box to give a high level of GA-regulated expression, and that together these motifs form important components of a gibberellin response complex in high-pI alpha-amylase genes. The TAACAAA box also appears to be the site of action of ABA.(ABSTRACT TRUNCATED AT 250 WORDS); http://www.plantcell.org/

In dicots, iron (Fe) is acquired from the soil by IRT1 (IRON-REGULATED TRANSPORTER 1) and FRO2 (FERRIC REDUCTION OXIDASE 2) that are localized at the root epidermis. IRT1 and FRO2 expression is induced by local and systemic signals under Fe-deficient conditions in Arabidopsis thaliana. In this study, the expression of IRT1, FRO2, bHLH038 and bHLH39 (the latter two of which control IRT1 and FRO2 expression) was promoted by GA4 treatment of gibberellin (GA) deficient ga3ox1 ga3ox2 mutants. In contrast, the expression of FIT, which encodes a transcription factor necessary for IRT1 and FRO2 induction under Fe deficiency, was not induced by the application of GA4. The induction of those genes triggered by shoot-applied GA4 was observed, even in the fit-2 mutant which had reduced endogenous GA levels caused by treatment with paclobutrazol (PBZ), a GA biosynthesis inhibitor. These results suggested that FIT was not a key regulator in the GA responses under Fe-sufficient conditions. On the other hand, among Fe uptake-related genes, the expression of IRT1, bHLH038 and bHLH39 was lower in ga3ox1 ga3ox2 compared with the wild type (WT) under Fe-sufficient conditions, but the expression of all Fe uptake-related genes decreased under Fe-deficient conditions. Additionally, the PBZ treatment decreased IRT1 expression in the WT under Fe-deficient conditions, but not in the fit-2 mutant. These data suggest the contribution of GA to the induction of Fe uptake-related genes under Fe-sufficient and Fe-deficient conditions, possibly in FIT-independent and FIT-dependent manners, respectively. http://www.plantphysiol.org/

GARE consensus (TAACARA, R = G or A) may be a Myb-binding site, isolated a GA-responsive Myb protein (GAMyb).
GARE sequences (GGCCGATAACAAACTCCGGCC
CaMV 35 S, and maizeUbi1 identified the following similar sequences:Amy1/6-4, −148GGCCGATAACAAA-CTC;Amy2/32b, −130 TCTC-GTAACAGA-GTC; CaMV 35 S, −503GGAC-CTAACAGAACTC; maizeUbi1, −312GGCGTTTAACAGG-CTG.
HRT –repressor of a-amylase gene- binds to GARE element; a novel DNA binding protein zinc finger transcriptional repressor. sequences: Amy1/6-4, −148GGCCGATAACAAA-CTC;Amy2/32b, −130 TCTC-GTAACAGA-GTC; CaMV 35 S, −503GGAC-CTAACAGAACTC; maizeUbi1, −312GGCGTTTAACAGG-CTG (bottom strand). Further analyses are required to determine whether HRT binds these or other sequences in the CAMV 35 S and maize Ubi1promoters. http://www.jbc.org/content;
http://www.ncbi.nlm.nih.gov/; ). http://www.jbc.org/content
In Barley GA response elements are located downstream of –174 and -108 and+54 are found to be GA and ABA response elements; -- -169pyrimidine box, -143TAACAAA box, and -124TATCCAC box-; In barley aleurone layers, the expression of genes encoding α-amylases and proteases is induced by GA but suppressed by ABA. It has been shown that an ABA-induced protein kinase, PKABA1, mediates the ABA suppression of α-amylase expression. The sequence of the central element, TAACAAA, is very similar to the c-Myb and v-Myb consensus binding site. Our results indicate that the GAMyb is the sole GA-regulated transcription factor required for transcriptional activation of the high-pI alpha-amylase promoter. We therefore postulate that GAMyb is a part of the GA-response pathway leading to alpha-amylase gene expression in aleurone cells. During embryogenesis antagonism between GA and ABA is very important in regulating the development transcription from embryogenesis to seed germination. In barley aleurone layers, the expression of genes encoding α-amylases and proteases is induced by GA, but suppressed by ABA;
(Gubler F, KallaR, RobertsJK, JacobsenJV.www.ncbi.nlm.nih.gov/pubmed)
Gibberellin (GA) response is complex in the region between -174 and -108: The sequence of the central element, TAACAAA, is very similar to the c-Myb and v-Myb consensus binding site. We investigated the possibility that a GA-regulated Myb transactivates alpha-amylase gene expression in barley aleurone cells. http://www.plantcell.org/
A cDNA clone, GAmyb, which encodes a novel Myb, was isolated from a barley aleurone cDNA library. RNA blot analysis revealed that GAmyb expression in isolated barley aleurone layers is up-regulated by GA.
The kinetics of GAmyb expression indicates that it is an early event in GA-regulated gene expression and precedes alpha-amylase gene expression.
GAMyb activates transcription of a high-pI alpha-amylase promoter fused to a beta-glucuronidase reporter gene in the absence of GA. Our results indicate that the GAMyb is the sole GA-regulated transcription factor required for transcriptional activation of the high-pI alpha-amylase promoter. We therefore postulate that GAMyb is a part of the GA-response pathway leading to alpha-amylase gene expression in aleurone cells.
it is now known that a-amylase isozymes are encoded by a family of 10 genes located on five different chromosomes (8-10). http://www.pnas.org/; In barley 7 genes encoding a-amylase isozymes map to chromosomes 1 and fourlow isoeleric point found on chromosome 6; there are several isozyme homologs for a-amylase in plants. http://www.ncbi.nlm.nih.gov/
Plants have 17 a-amaylase genes. Results indicate that the cereal alpha-amylase genes fall into two major classes: AmyA and AmyB. The AmyA class(Barley and whaet) is subdivided into the Amy1 and Amy2 subfamilies previously used to classify alpha-amylase genes in barley and wheat. The AmyB class three sub familyincludes the Amy3 subfamily to which most of the alpha-amylase genes of rice belong(Rice). a-Amyl genes reside on homeologous chromosomes 6A, 6B, and 6D, whereas 10 or 11 a-Amy2 genes are found on chromosomes 7A, 7B, and 7D. The wheat a-Amy3 genes map to the homeologues of chromosome 5. Different experimental approaches to the study of cereal,
small gene family-49kDa
Effect on Vernalization:
Not all seeds released from the fruits germinate immediately. Some pass through a period of dormancy. And those seeds which exhibit temporary suspension of growth activity are called dormant seeds. In order to make dormant seeds germinate they have to be subjected to scarification or stratification or some of them have to be subjected cold treatment. Plants or seeds acquiring the ability to accelerate flowering in response to cold treatment is called vernalization.
GA is known to overcome cold treatment for plants that requires vernalization, but it can also break the seed dormancy. Quantitative estimation of GA in vernalized seeds do not show any increase in GA content in response to cold treatment, however, during germination at normal temperature, the concentration of GA increases significantly; these studies suggest that cold treatment per se does not increase GA content, but provides the ability to synthesize more GA during germination and growth. In some instances, where dormant seeds requiring far-red treatment to break the dormancy GA acts as the substituent.
Effect on Parthenocarpy:
Where auxins fail, GAs are found to be very effective in inducing Parthenocarpy.
In addition to it, GA induced parthenocarpic fruits are larger in size and sweeter
in content. GA is used to increase the total yield of grape fruits. It is
also highly effective in increasing the yield and sugar content of sugar cane. However,
the application of GAs should not be more than what is required in grape
cultivation; otherwise fruits will be damaged
.

Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor; www.pnas.org
Gibberellins and Auxin Interactions:
Gibberellins and auxins are found to induce cell elongation, parthenocarpy and metabolic activities including RNA and protein synthesis. But GA and auxins have their own specific effects on different tissues of the plant body. While GA promotes internode elongation, overcomes genetic dwarfism, induces amylase synthesis in aleurone cells; it can substitute cold treatment or far red treatment. IAA does not elicit any of these effects. On the other hand, auxins impose apical dominance, induce adventitious roots and induce cellulase synthesis. On the other hand GAs doesn’t elicit any of the auxins’ responses. However, in the case of cell elongation though GA and IAA have independent actions in promoting the growth of etiolated normal pea stem sections, if both the hormones are provided together, their total effect is just additive but not synergistic. On the contrary, if internodes of dwarf pea stems are treated with either GA or IAA, the promotive effect on stem segments is very little, but if both are provided together the stimulation in terms of growth is highly pronounced and the total effect is synergistic. The above observation indicates that gibberellins need auxins for synergistic activity. This particular conclusion is further substantiated by the fact that a decapitated internodal segment does not respond to GA treatment alone but if the apical meristem or an agar block containing auxin is placed on the decapitated segment, elongation of the internode is stimulated in the presence of GA. This is because apical meristems do synthesize auxins, which interact with gibberellins to bring about the combined effect. Recent studies in in vivo and invitro system strongly suggest that GA has an important role in promoting the biosynthetic pathway of auxin, thus in the presence of GA, the levels of auxins increase. Probably GAs enhances the rate of IAA synthesis.


www.5e.plantphys.net
Extraction of pea seedlings shows that decapitation reduces endogenous GA1 content of plants after 48 hours. IAA (auxin) applied at time 0 to a decapitated stump (arrow) restores GA1 levels after 48 hours, and maintains elongation of the uppermost intact internode to the same level as that in the intact plant. The uppermost intact internode in the decapitated seedling elongates slightly less (about 10%) during the 48 hour duration of the experiment.
IAA (from the apical bud) promotes and is required for GA1 biosynthesis in subtending internodes of pea. IAA also inhibits GA1 breakdown.
One of the mechanisms to coordinate growth and development in both vegetative and reproductive organs of plants is through auxin stimulation of GA biosynthesis and deactivation. Further research will clarify how multiple hormonal interactions modify vegetative and reproductive growth and development, which may lead to the development of new cultivars of crop plants or modification of cultural practices for more efficient and sustainable crop production.

Junaid Ziauddin and David M. Sabatini; http://www.nature.com/
a, Protocol for making microarrays of transfected cells. b, Laser scan image of a
GFP-expressing microarray made from a slide printed in a 14 ![]() 10 pattern with a GFP expression construct. c, Higher magnification image
obtained with fluorescence microscopy of the cell cluster boxed in b. Scale bar, 100
10 pattern with a GFP expression construct. c, Higher magnification image
obtained with fluorescence microscopy of the cell cluster boxed in b. Scale bar, 100 ![]() m. d,
Expression levels of cell clusters in a microarray are proportional, over a
fourfold range, to the amount of plasmid DNA printed on the slide. Indicated
amounts of the GFP construct assume a 1-nl printing volume. The graph shows the
mean
m. d,
Expression levels of cell clusters in a microarray are proportional, over a
fourfold range, to the amount of plasmid DNA printed on the slide. Indicated
amounts of the GFP construct assume a 1-nl printing volume. The graph shows the
mean ![]() s.d. of the fluorescence intensities of the cell
clusters (n = 6). The
fluorescent image is from a representative experiment. e, Co-transfection is possible
with transfected cell microarrays. Arrays with elements containing expression
constructs for HA–GST, GFP or both were transfected and processed for immunofluorescence
and imaged with a laser scanner. Cy3, cell clusters expressing HA–GST; GFP,
cell clusters expressing GFP; merged, superimposition of Cy3 and GFP signals.
Yellow colour indicates co-expression. Scale bar, 100
s.d. of the fluorescence intensities of the cell
clusters (n = 6). The
fluorescent image is from a representative experiment. e, Co-transfection is possible
with transfected cell microarrays. Arrays with elements containing expression
constructs for HA–GST, GFP or both were transfected and processed for immunofluorescence
and imaged with a laser scanner. Cy3, cell clusters expressing HA–GST; GFP,
cell clusters expressing GFP; merged, superimposition of Cy3 and GFP signals.
Yellow colour indicates co-expression. Scale bar, 100 ![]() m. f,
Enlarged view of boxed area of scan image from e.
m. f,
Enlarged view of boxed area of scan image from e.
Genome and expressed sequence tag projects are rapidly cataloguing and cloning the genes of higher organisms, including humans. An emerging challenge is to rapidly uncover the functions of genes and to identify gene products with desired properties. We have developed a microarray-driven gene expression system for the functional analysis of many gene products in parallel. Mammalian cells are cultured on a glass slide printed in defined locations with different DNAs. Cells growing on the printed areas take up the DNA, creating spots of localized transfection within a lawn of non-transfected cells. By printing sets of complementary DNAs cloned in expression vectors, we make microarrays whose features are clusters of live cells that express a defined cDNA at each location. Here we demonstrate two uses for our approach: as an alternative to protein microarrays for the identification of drug targets, and as an expression cloning system for the discovery of gene products that alter cellular physiology. By screening transfected cell microarrays expressing 192 different cDNAs, we identified proteins involved in tyrosine kinase signalling, apoptosis and cell adhesion, and with distinct subcellular distributions. Note-The same method above can be used to find GA induced Gene expression by microarray method
|
Effects |
IAA |
GA |
|
Stem Growth |
+ |
+ |
|
Parthenocarpy |
+ |
+ |
|
Root initiation |
+ |
- |
|
Callus formation |
+ |
- |
|
Induction of amylase |
- |
+ |
|
Bolting and flowering |
- |
+ |

GA and ABA:
Plant hormonal interactions are fascinating for the simple reason that many of them act antagonistically and some cooperatively and few synergistically. Some of the interactions are concentration dependent. GA and Auxin induce parthenocarpy; Cytokinins and auxins act antagonistically in Auxin induced new root formation. Induction of dormancy and breaking dormancy are two opposite development pathways in seeds. ABA can induce dormancy and GA can break the dormancy. Cold treatment induces vernalization in some plants in ABA dependent manner, but vernalization can be broken by GA.

The diagram illustrates how ABA and GA act in opposite ways in response to environmental inputs.
GA and Flowering:
Plants which require longer photoperiodic conditions for flowering are called long day plants and those plants requiring shorter photoperiod are called short day plants. Long day plants do not flower under short day conditions and vice versa. Correct photoperiod treatments stimulate the synthesis of flowering hormone called florigin which inturn induces flowering by differential gene expression. However, there are a large number of plants which do not require such photoperiodic conditions for flowering, they flower as they grow and mature; may be called Day-Neutral pants.

FCA and the control of Arabidopsis flowering time. (A and B) Schematic representation of the principal genetic pathways controlling flowering time in winter annual and rapid cycling accessions of Arabidopsis. Promotive activities are denoted by arrowheads, repressive activities are denoted by T-bars. The photoperiod (PP) and gibberellin signal transduction (GA) pathways are shown activating genes with a floral meristem identity function (FMI), while FLC is shown to repress this. FRI, the FCA-containing autonomous pathway (AUT) and vernalization pathway (VRN) regulate FLC in an antagonistic manner. The most frequently found difference between winter annual (A) and rapid cycling (B) Arabidopsis accessions is allelic variation at FRI, with most rapid cycling accessions carrying inactive, loss-of-function fri alleles. (C) A comparison of the phenotype of wild-type Ler plants and late flowering fca-1 mutant plants. Although grown for the same time and under identical conditions, wild-type plants have already flowered while the fca-1 plants have remained in the vegetative state and continued to produce more leaves as opposed to floral organs. (D) Schematic representation of the alternative processing of Arabidopsis FCA pre-mRNA. Exons are represented as filled boxes and intron by lines.
Some of the long day plants under non inductive short day conditions exhibit rosette leaves because of extreme condensation of internodes. If such plants under non inductive conditions are sprayed with Gibberellins, they get stimulated and the internodes elongate dramatically; such GA induced elongated stems also initiate flowering, such a phenomenon is known as Bolting and Flowering. Though bolting and flowering are two different events, GA has been found to effect the elongation of internodes first during which process flowering substances are also produced as a result floral buds are initiated.

www.imgarcade.com
Furthermore, quantification of GA in untreated plants and plants which are in flowering show very low amount of GA in the former and higher content in the later. It amounts to the fact that during proper photoconductive conditions synthesis of Gibberellins increases. However, GA has no effect on majority of short day plants. Strangely particular variety of short day rice plants responds very well to GA treatment and produce flowers.
From the above observations, it is clear that Gibberellins actually overcome plants requiring long day photoperiod. The interrelationship between long day photoperiodic treatments and GA effect has been explained on the basis of the following factors. Plastids synthesize the flower inducing substance and store the same under long day photo periodic condition. At the same time, the phytochrome pigments which are also present within plastids, absorb red light and transform them into far red light absorbing pigment. This form of pigments labializes the plastid membranes and thus Gibberellins are released from the plastids. Thus the concentration of GA increases in photo period induced long day plants. In spite of it, the exact mechanism by which GA brings about differential gene expression during flowering is not clear. Probably, it may also require other factors for floral induction.
Effect on inflorescence and floral structure:
Though GA is known to induce bolting and flowering in long day plants, the effect of GA on a short day plants like sorghum bicolor is very interesting. In these plants, GA3 stimulates flowering even under non inductive conditions. But in combination with far red light treatment GA’s effect is synergistic. The hormone also brings about marked increase in the number of spikelets and glumes. Along with these promotive responses, GA also increases the number of floral structures like ovaries and promotes fertilization, but inhibits stamen development. Effect on flower induction:


In the presence of GA the repressors are destroyed and allow PIFs to bind to their respective promoter elements and activate specific genes needed flower induction. Schematic representation of events leading to GA-induced floral transition in Arabidopsis. In the leaf, phytochrome-mediated up-regulation of GA20ox results in increased GA concentration, which may up-regulate FT, also under photoperiod control, via CO. GA and FT protein move from the leaf to the shoot apex. Inactivation of GA by GA2ox in the rib meristem regulates the amount of GA entering the shoot apical meristem, where it activates SOC1 and LFY via repression of the DELLAs GAI and RGA. Red arrows (promotion) and T-bars (repression) indicate steps that immediately affect or are affected by GA. Boxed numbers refer to supporting data for the represented scheme as follows: (1) Achard et al., 2004; (2) Liu et al., 2008; (3) Leeet al., 2008; (4) Liu et al., 2007; (5) Gocal et al., 2001; (6) Hisamatsu and King, 2008; (7) Hisamatsu et al., 2005; (8) Jasinski et al., 2005. * Authors presented data to show that these two pathways are independent. http://jxb.oxfordjournals.org/
“Effects of GAs on Flowering and Flowering Genes”
“By Valerie Sponsel, Biology Department, University of Texas, San Antonio, TX, USA, ‘with acknowdgment”
Flowering in Arabidopsis, a plant for which more tools exist to study the molecular genetics of the process than in any other species, is regulated by four separate pathways: (a) the photoperiodic (long day) pathway, which operates in the leaves; (b) the convergent autonomous (leaf number)/vernalization (low temperature) pathway; (c) the carbohydrate (sucrose) pathway; and (d) the gibberellin pathway. The latter three pathways all operate in the shoot apical meristem. The four pathways converge on a number of floral pathway integrators that together regulate floral initiation.
The recent identification of FLOWERING LOCUS T (FT) as the gene expressed in leaves that encode a phloem-mobile mRNA or protein that fits the description of a universal flowering stimulus called ‘Florigen’, an elusive molecule that perplexed plant scientist for at least a century or so. Discovery of florigen protein has been an exciting development after the decades-long search for this elusive flowering substance (Huang et al. 2005; and see Zeevaart 2006, for a review). Additional work is seeking to further define the integrative networks that exist between the different pathways. Web Essay 25.2, from which Figure 1 is taken, provides a complete consideration of this subject. This topic briefly addresses the GA pathway, when it operates, and what is known about how it is integrated with the other pathways.

|
|
|
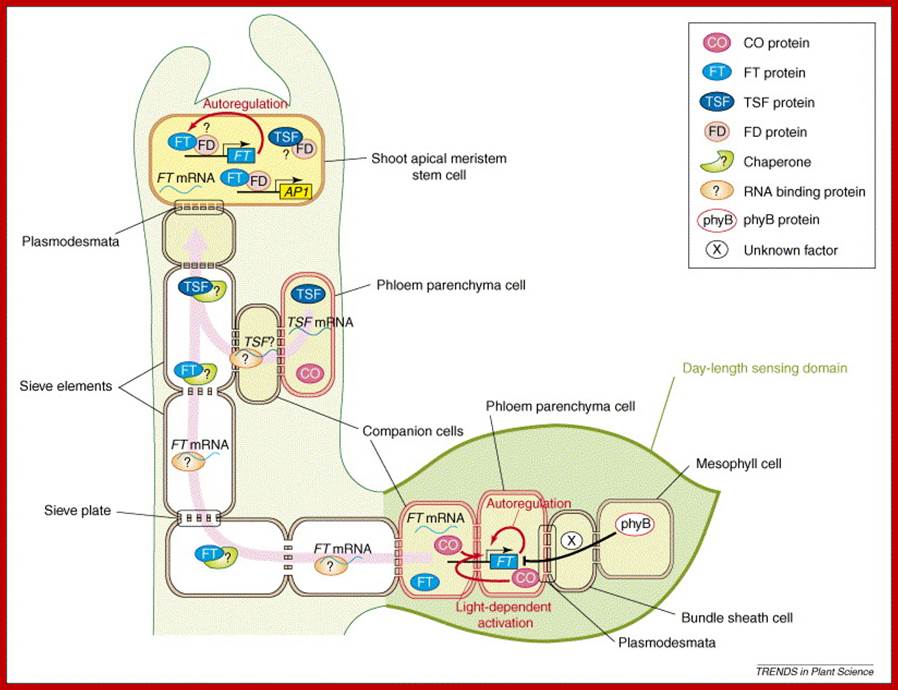
Multiple developmental pathways for flowering in Arabidopsis: photoperiodism, the autonomous (leaf number) and vernalization (low temperature) pathways, the energy (sucrose) pathway, and the gibberellin pathway. The photoperiodic pathway is located in the leaves and involves the production of a transmissible floral stimulus, FT protein. In LDPs such as Arabidopsis, FT protein is produced in the phloem in response to CO protein accumulation under long days. It is then translocated via sieve tubes to the apical meristem. In SDPs such as rice, the transmissible floral stimulus Hd3a protein accumulates when the repressor protein, Hd1, is not produced under short days, and the Hd3a protein is translocated via the phloem to the apical meristem. In Arabidopsis, FT binds to FD, and the FT/FD protein complex activates the AP1 and SOC1 genes, which trigger LFY gene expression. LFY and AP1 then trigger the expression of the floral homeotic genes. The autonomous (leaf number) and vernalization (low temperature) pathways act in the apical meristem to negatively regulate FLC, a negative regulator of SOC1. The sucrose and gibberellin pathways, also localized to the meristem, promote SOC1 expression. (After Blázquez 2005.) (Click image to enlarge.) plant cell biol; www.5e.plantphys.net
The nature of floral signals in Arabidopsis. Roles of FLOWERING LOCUS T (FT) and gibberellin; Hisamatsu T, King RW - J. Exp. Bot. (2008); www.openi.nlm.nih.gov |
|
|
|
http://gcat.davidson.edu/ |
|
|

- Mechanism of
flowering by binding between florigen and
its receptors in shoot apical cells; world’s first discovery of receptors of flowering hormone ‘Florigin’. Prof.Ko Shimoto, Nara institute of science And technology.
Flowering induction in plants occurs as follows. Florigen synthesized in the leaf is transported through the vascular bundle to the shoot apex, from which flowers come out. In the shoot apical cells, florigen binds to the 14-3-3 receptor in the cytoplasm. The resulting Hd3a-14-3-3 complex is transported into the nucleus and binds to OsFD1 to form a higher complex, the florigen activation complex. This complex binds to the promoter of genes that induce the formation of flower buds, and activates the genes that in turn activate a series of genes that promote flowering. http://www.spring8.or.jp/ www.spring8.or.jp

Crystal structure of florigen activation complex; world’s first florigin receptor. The crystal structure of the florigen activation complex, which is a hexamer comprising two each of three proteins, i.e., Hd3a, 14-3-3, and OsFD1, and is responsible for the activation of florigen, was determined. DNA-binding sites were simulated by modeling techniques. Red, blue, and green ribbon diagrams represent florigen, the 14-3-3 protein (receptor), and the OsFD1 transcription factor that binds to DNA, respectively. Nara Institute ofScience and Techanology; http://www.spring8.or.jp/
Photoperiodic control of flowering; The timing of floral transition has a direct impact on reproductive success. One of the most important environmental factors that affect the transition is the change in day length (photoperiod). Classical experiments imply that plants monitor photoperiods in the leaf, and transmit that information coded within an elusive signal dubbed florigen to the apex to reprogram development. Recent advances in Arabidopsis research indicate that the core of the day-length measurement mechanism lies in the circadian regulation of CONSTANS (CO) expression and the subsequent photoperiodic induction of the expression of FLOWERING LOCUS T (FT) gene, which might encode a major component of florigen. In this review, we introduce current perspectives on how, when and where the floral signal is generated. Takato Imaizumi; Steve A. Kay; http://www.cell.com/
In Arabidopsis, flowering occurs in long days (LD) even in the GA-deficient ga1-3 mutant although it is somewhat delayed compared to wild-type plants. However, this mutant will not flower in short days (SD) unless treated with GA. Double mutants of ga1-3 and co, (the wild-type CONSTANS is normally expressed in leaves and is upstream of FT in the LD pathway) will not normally flower, either in SD or LD. From this and other information it is proposed that the LD and GA pathways are independent, with the GA pathway being essential for flowering in noninductive conditions. Input from the LD pathway (transduced through FT) and from the GA pathway converge on SOC 1 (SUPPRESSOR OF OVEREXPRESSION OF CO 1), that is expressed in the apical meristem. Thus, GA treatment to ga1-3 mutants in SD causes both enhanced expression of SOC in the apical meristem, and flowering. Over expression of SOC1 in ga1-3 mutants allows them to flower in SD without GA treatment (Moon et al. 2003).
An additional floral pathway integrator is LFY, the product of a floral meristem identity gene that is expressed in apical meristems. LFY expression, which is high in LD-grown plants, is only observed in SD-grown ga1-3 mutants that have been treated with GA. Constitutive LFY expression will also allow SD-grown ga1-3 to flower without GA treatment (Blazquez et al. 1998). Using deletion analysis, Blazquez and Weigel (2000) identified an 8 bp motif in the LFY promoter that is necessary for GA responsiveness, but not for response to day length. This sequence is a potential target for MYB transcription factors and candidate MYBs whose expression increases in the shoot apex in response to exogenous GA4, or to elevated levels of native GA4, have been identified in both Lolium temulentum and Arabidopsis (Gocal et al. 1999, 2001b). Interestingly, LDs—but not GA treatment—cause a rapid increase in expression of the L. temulentum ortholog of FT (LtFT), indicating in this plant too that the LD and GA pathways converge downstream of FT (King et al. 2006).

In the 1930s, the flowering hormone, florigen, was proposed to be synthesized in leaves under inductive day length and transported to the shoot apex, where it induces flowering. More recently, generated genetic and biochemical data suggest that florigen is a protein encoded by the gene, FLOWERING LOCUS T (FT). A rice (Oryza sativa) FT homolog, Hd3a, interacts with the rice FD homolog, OsFD1, via a 14-3-3 protein. Formation of this tri-protein complex is essential for flowering promotion by Hd3a in rice. In addition, the multifunctionality of FT homologs, other than for flowering promotion, is an emerging concept. Here we review the structural and biochemical features of the florigen protein complex and discuss the molecular basis for the multifunctionality of FT proteins. www.cell.com