Plant Hormones-AUXINS
Distribution
Though auxin is synthesized in the plant apices of shoots and roots, it is transported towards their respective basal parts. Quantitative estimation of auxins found in the segments of seedlings, by spectrophotometric analysis; show that stem apex possesses 1.5 to 2.0 fold higher amounts than found in the root apices. The lowest concentration is found in the region of the stem where cotyledons are attached. Even leaves contain some amount of Auxins.
Evolution has provided at least two particularly successful independent solutions to the problems of multicellularity — animals and higher plants. An obvious requirement for successful multicellularity is communication between different parts of the organism, both locally, for example between neighboring cells, and over very long distances. Recent advances in understanding hormone signalling networks in plants are beginning to reveal how co-ordination of activity across the whole plant body can be achieved despite the lack of a control center, typical of animal systems. Of particular importance in this distributed regulatory approach are the self-organizing properties of the transport system for the plant hormone auxin. This review examines the integrative role of the auxin transport network in co-ordinating plant growth and development. Ottoline Leyser; http://www.cell.com/
Auxin transport from the site of synthesis to site of action; /www.psla.umd.edu

ABCBs (P-glycoproteins) and auxin transport ATP binding family (ABCBs) are PM anion transporters characterized by 12 membrane-spanning helices, 2 ATP-binding sites, a phosphorylation site, and a C-terminal protein-protein interaction domain. The 27 ABCBs in Arabidopsis each have tissue-specific expression & localization. ABCBs were initially associated with auxin transport when they were purified by affinity chromatography with NPA, an auxin efflux inhibitor; https://www.psla.umd.edu
Looking at the structure, functional responses, plants are no different from animals’ system neuronal network. Holistically one can imagine plants having a system are no different from animal systems.
Symposium on plant Neurobiological view of plants 2005; www.ds9.botanik.uni-bonn.de
Root apex and stem apex– THE ANTERIOR and posterior POLE s OF PLANT BODY-sensor perception or receiving structures; AUXIN – PLANT NEUROTRANSMITTER, CELLULAR END-POLES – PLANT SYNAPSES; VASCULAR STRANDS – PLANT NERVES, ROOT APICES INTERCONNECTED VIA VASCULAR CYLINDERS – SERIAL NERVOUS SYSTEM OF PLANT; Plant Signaling and Behavior describes a growing, but also old and fascinating field in plant biology addressing the physiological and neurobiological basis of adaptive behavior in plants. The word neuron was coined by German scientist Heinrich Wilhelm Gottfried von Waldeyer-Hartz in 1891 and originates from the Greek meaning string or sinew according to the Oxford English Dictionary. Franti sek Balu ska says the word comes from the Greek meaning vegetal fiber. "Maybe it's time to steal it back."

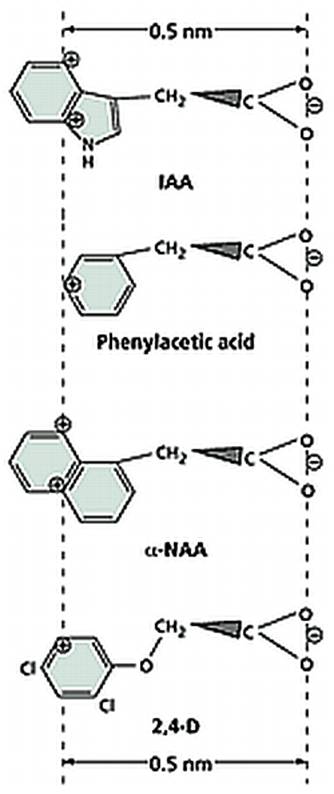
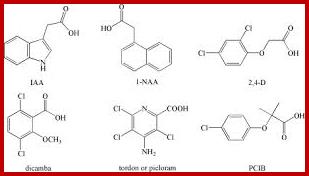
Chemical structures of few Natural and Synthetic auxins; http://openi.nlm.nih.gov/
Auxins exist either in bound form or in a free state. The non diffusible form is considered as bound form and it is active, but the freely diffusible form is referred to as an inactive form. The bound state of the auxin has been explained as due to the binding of receptor proteins or binding proteins to auxins. Such proteins are found in the plasma membranes, cytoplasm and chromosomes. The mol. wt. of auxin binding proteins has been determined as 10,000 and 31, 5000 Daltons. Such proteins have been isolated from the free nuclei of coconut liquid endosperm. These proteins in the presence of IAA are found to be active in inducing gene expression. In living cells, the relative concentrations of bound form and free forms of auxin is not constant but depends upon the environmental conditions or the functional state of cells.
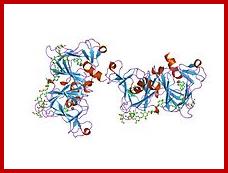
Crystal structure of auxin-binding protein 1 in complex with 1-naphthalene acetic acid;
http://en.wikipedia.org/
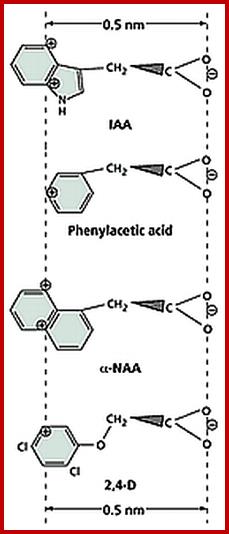
Auxins with COO (+/-) charged at one end
Site of synthesis:
The natural auxin found in plants is called Indole Acetic Acid (IAA) and it is the first of the phytohormones to be discovered. Indole acetic acid is predominantly synthesized in the stem apexes. Using radioactive 14C as the tracer, it has been demonstrated that the meristematic cells, just above differentiating into vascular tissues, are found to be active in synthesizing IAA. Shoot apexes synthesize more auxin than the root apecies.
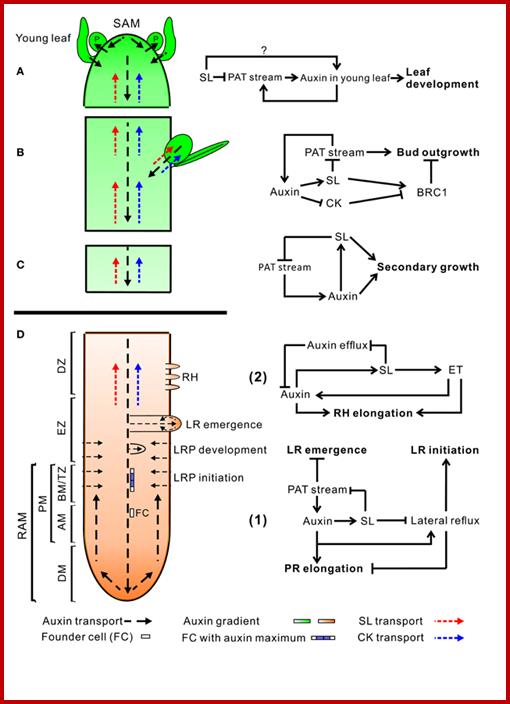
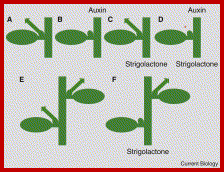
An overview of auxin, SL, and CK transport within the plant (left) and hormone interactions during the regulation of shoot and root development (right). Auxin, strigolactone (SL), and cytokinin (CK) transport are represented by black, red, and blue dotted line, respectively. For hormone interactions (right), arrows represent promotion, while flat-ended lines indicate inhibition. (A)Auxin, produced in the shoot apical meristem (SAM) and young leaves, is transported basipetally through the stem in the polar auxin transport (PAT) stream toward the root apical meristem (RAM). Here, but probably also throughout the entire vasculature of the plant, it positively regulates SL biosynthesis (Hayward et al., 2009). As shown by GR24 feeding experiments, SLs transported through the xylem from the root to the shoot down-regulate the free auxin level in young leafs in a MAX2-dependent manner hereby controlling their development (Ruyter-Spira et al., 2011). SLs in the vasculature negatively affect PAT capacity (Crawford et al., 2010), as observed for NPA (Ljung et al., 2001), which negatively feeds back on auxin levels at the sites of biosynthesis. This long distance SL-auxin feedback mechanism, affects plant developmental processes as described below. (B) During the regulation of bud outgrowth, SLs reduce the capacity of the PAT stream in the main stem, leading to enhanced competition between buds to release their auxin into the stem (Crawford et al., 2010; Shinohara et al., 2013). On the other hand, SLs and CK are transported acropetally through the xylem and act directly in the buds to control their outgrowth through the joint regulation of TCP transcription factor BRC1 (Braun et al., 2012; Dun et al., 2012). (C) SLs have a direct positive effect on secondary growth by activating cell division in the vascular cambium in which they act downstream of auxin. The fact that the max1 mutant still displays some residual cambium activity might point to a SL independent response to auxin. However, this remaining activity could also be due to residual SLs in these mutants (Agusti et al., 2011). (D) Hormone interactions during primary root (PR) elongation, lateral root (LR) initiation and development (1) and root hair (RH) elongation (2). (1) Auxin imported from the main PAT stream into the root stimulates SL production. SL export into the xylem and down regulation of the PAT stream feedback on auxin levels in the shoot as described under (A). SL biosynthesis genes are specifically expressed in vascular tissue and the cortex of the proximal meristem of the root, through which the lateral auxin reflux toward the main PAT stream takes place. Therefore it is likely that locally synthesized SLs are controlling the efficiency of this reflux. Primary root elongation and lateral root initiation are determined by the auxin gradient inside the root tip, which is determined by auxin levels imported through the PAT stream, auxin synthesized in the root tip, and local auxin transport, including the auxin lateral reflux. Lateral root development and emergence are controlled by auxin derived from the shoot for which the SL controlled PAT stream capacity and lateral auxin influx into the developing lateral root primordia (LRP) are the main determinants. Although in the flow diagram auxin is depicted as a positive regulator of root growth, auxin displays a dose-response curve with an optimum, such that supra-optimal auxin concentrations will have a negative effect (Ruyter-Spira et al., 2011). (2) The effect of SLs on RH elongation is dependent on both auxin and ethylene (ET) biosynthesis and signaling. It has been suggested that SLs negatively regulate auxin efflux (Koltai et al., 2010). If this would specifically occur in RH cells this would result in increased local auxin levels which stimulates RH elongation. This local action of SLs has not been proven yet. Alternatively, it may be that SLs affect auxin transport in the PAT stream and/or the root tip hereby indirectly affecting the auxin concentration in RH cells. ET acts downstream of SLs and has a direct effect on RH elongation but also interacts with the auxin pathway (Kapulnik et al., 2011b). Abbreviations: P, primordium; DM, distal meristem; PM, proximal meristem; AM, apical meristem; BM, basal meristem; TZ, transition zone; EZ, elongation zone; DZ, differentiation zone; FC, founder cell.Auxin synthesized at the step apex and cytokinins are produced at root apex then they are transported to opposite direction i.e. towards their basal regions; www.all-science-fair-projects.comhttp://www.frontiersin.org/
Biosynthesis of IAA:
Indole Acetic Acid IAA is biosynthesized in tryptophan dependent and tryptophan independent pathways. In Tryptophan dependent pathway it runs through four different pathways. Tryptophan has been found to be the precursor for IAA synthesis. In some cases Indole acetonitrile has also been found to be used in the synthesis of IAA. The enzymes responsible for the biosynthesis of IAA have been identified. There are two pathways which converge in the production of IAA. To start with, tryptophan is converted to Indole pyruvate by deamination reaction catalyzed by specific deaminase enzymes. The indole 3D pyruvate is then subjected to decarboxylation to produce indole acetaldehyde, which is then oxidized by alde-hydrases to indole 3- acetic acid. On the other hand, tryptophan may be first subjected to decarboxylation step. The tryptamine synthesized in this reaction is deaminated to indole 3- acetaldehyde then it is oxidized to IAA.
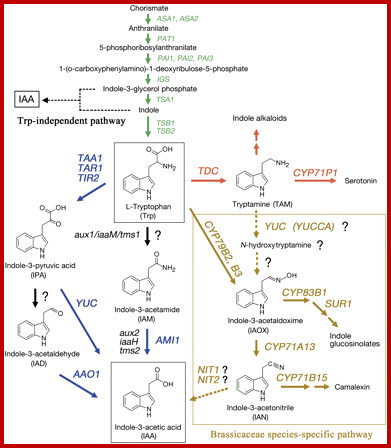
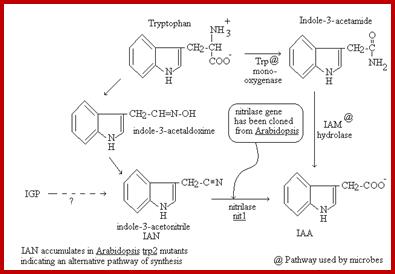
Presumptive pathways for IAA biosynthesis in plants; Green arrows indicate the tryptophan synthetic pathway in the chloroplast. A thin dashed black arrow denotes the tryptophan-independent IAA biosynthetic pathway. Blue arrows indicate steps for which the gene and enzymatic function are known in the tryptophan-dependent IAA biosynthetic pathway. Red arrows indicate the indole alkaloid and serotonin biosynthetic pathway. Mustard-coloured arrows indicate the Brassicaceae species-specific pathway. Black arrows indicate steps for which the gene(s) and enzymatic function(s) are unknown. Dashed mustard-coloured arrows indicate steps for which the gene and enzymatic function(s) remain poorly understood. Letters in italics show genes involved in the conversion process. Lower case letters in italics indicate bacterial genes. http://jxb.oxfordjournals.org/ Alternative IAA synthesis-www.hort.purdue.edu
Synthetic Auxins:
Once the native auxin has been identified as indole acetic acid, plant biochemists started looking for similar compounds in nature as well as in the laboratory. As a result, a host of synthetic auxins have been discovered and their effects as growth promoting hormones have been characterized, ex. Indole propionic acid Indole butyric acid Naphthalene acetic acid, phenyl acetic acid, 2,4 Dichlorophenoxy acetic acid are just few of the known synthetic auxins.
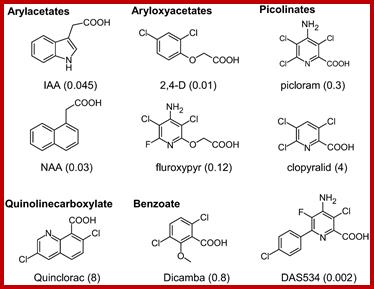
Synthetic Auxins; Plus.google.com; openi.nlm.nih.gov
Chemical structures of IAA, several commercial auxinic herbicides, and the experimental herbicide DAS534. The concentrations of herbicide (μM) required to produce a growth reduction of Arabidopsis roots by 50% relative to untreated controls (GR50) in our assay system are shown in parentheses. www.plantphysiol.org
The hormonal activity of IAA and the other synthetic compounds has been attributed to the presence of a ring system as the nucleus, a side chain possessing a carboxyl group and the presence of at least one carbon atom between the ring and the carboxyl end. The length of the side chain, the number of substituents in the nucleus and the side chain and the basic structure of the nuclear ring have been found to exert profound influence in eliciting physiological responses.
Auxin Transport:
Auxin is transported from the site of synthesis to the site of action, which is not far away. Nevertheless auxin is also translocated to other regions of the plant body. The transportation of auxin is polar, i.e., from apex to the base, which is called basipetal movement, but acropetal movement i.e. from the base to apex has also been observed but the amount transported is almost negligible. The ratio between basipetal and acropetal movement is approximately 3:1.
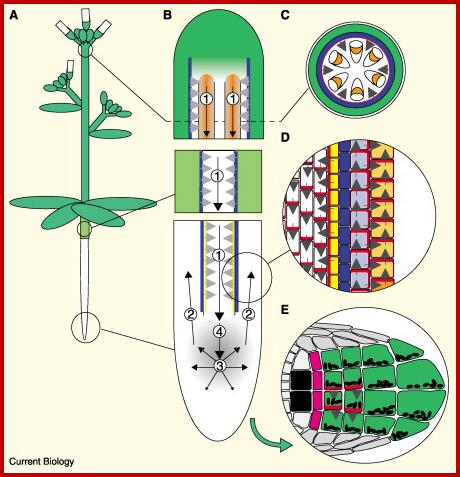
A model for auxin transport and redistribution during tropic stimuli, based on PIN protein localisation.
(A) Schematic diagram of an Arabidopsis plant. (B) Direction of polar auxin transport (black arrows), and the sites of action of PIN1–PIN4 (numbers), in the stem (top), hypocotyls (middle) and root (bottom). Stem vascular bundles and root meristem are indicated by orange and grey shading, respectively. The horizontal grey arrow respectively. The horizontal grey arrow heads indicate the predicted direction of auxin transport mediated by PIN3 in the endodermis (blue) and the root pericycle (yellow). (C) Cross-section of the stem showing the epidermis (white), cortical cells (green), endodermis (blue) and xylem parenchyma (orange) of the vascular bundles. The polarized distribution of PIN3 at the inner face of the epidermal cell layer is represented by the red ring. Grey arrows indicate the predicted direction of auxin efflux mediated by PIN3. (D) Schematic diagram of polarized PIN protein distribution (red) in individual root tissues: stele (white), pericycle (yellow) endodermis (blue), cortex (violet) and epidermis (orange). Arrows indicate the direction of auxin efflux from each cell. (E) Representation of PIN3 distribution (red) in a section through the root cap 5 minutes after reorientation with respect to gravity. Columella initials (pink), columella cells (green) with Amyloplasts (black ovals), quiescent center cells (black). http://www.sciencedirect.com/
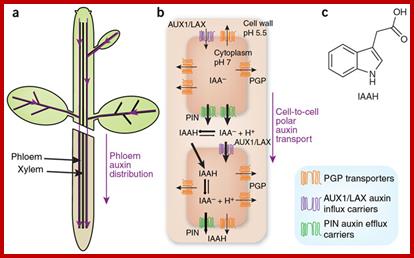
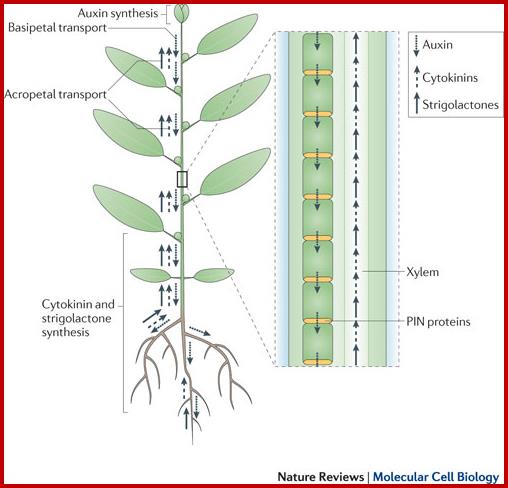
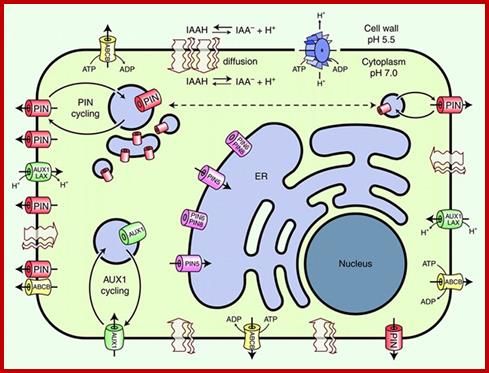
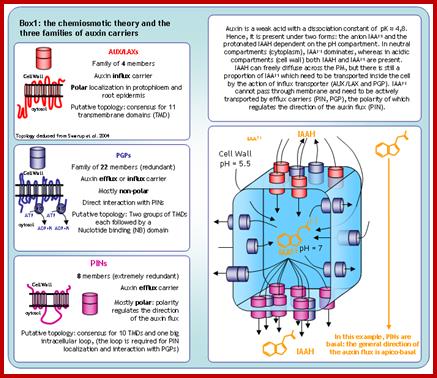
Phloem-based transport and chemiosmotic model for polar
auxin transport.; Auxin and other signals on the move in
plants; (a) Auxin distribution via the phloem from source tissues—young leaves
and floral buds—to root and shoot tips. (b) The chemiosmotic model,
based on the pH difference between the apoplast (pH 5.5) and the cytoplasm (pH
7.0). Protonated auxin—undissociated indole-3-acetic acid (IAAH)—can diffuse
through the lipidic plasma membrane or be transported by the AUX1/LAX influx
carriers into the cell. In the cytosol, it dissociates and gets trapped inside
the cell in its deprotonated form (IAA-). IAA- can exit cells by the action of PGP-
or PIN-type efflux carriers. The polar cellular localization of the carriers
determines the directionality of the intercellular auxin flow. (c) Structure of protonated IAAH. Hélène S Robert & Ji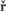 í Friml; www.nature.com
í Friml; www.nature.com
Auxins, cytokinins and Strigolactones (or Strigolactones derivatives) are three classes of hormones that are involved in the control of bud activation. These hormones are transported throughout the plant, forming a systemic network that allows integration of information between different plant organs. Auxin is mostly produced in the young expanding leaves of growing shoot apices and is actively transported basipetally in the polar auxin transport stream, involving basally localized PIN-FORMED (PIN)-type auxin efflux carriers, in particular PIN1. Strigolactones and cytokinins are mainly produced in the root, but also locally in the shoot, and are transported acropetally in the xylem. http://www.nature.com/
IAA enters (influx) parenchyma cells in a protonated form via passive transport, or as a anion through an influx carrier protein. Once inside the cell, IAAH dissociates because of the higher pH within the cytoplasm to become an anion. Ions are the only form of auxin that can leave the cell (efflux) through a efflux carrier protein. http://quizlet.com/
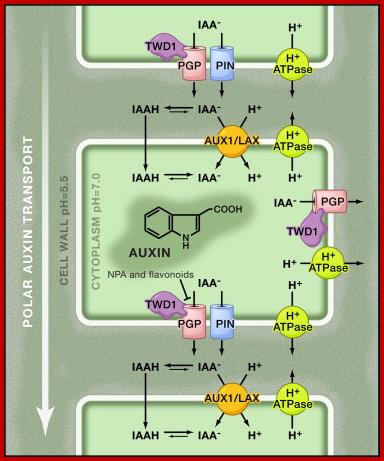
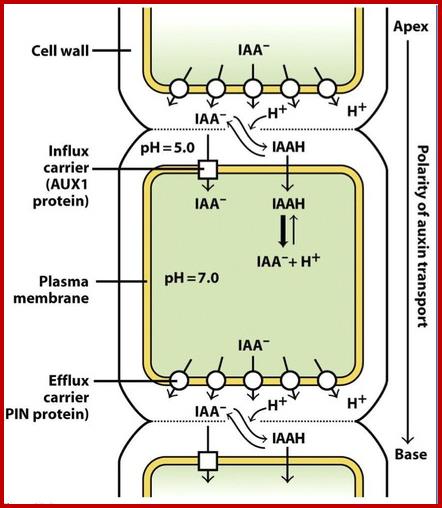
Chemiosmosis Hypothesis for Polar Auxin Transport:
The low pH in the apoplast (cell wall) is maintained through the activity of plasma membrane H+ ATPases. In the relatively acidic environment, a fraction of the weak acid, indole-3-acetic acid (IAA), the major form of auxin, becomes protonated. The protonated (IAAH) form is more lipophilic and can diffuse freely through the plasma membrane into the cell. Besides passive diffusion, auxin is also actively taken up from the apoplast by H+/IAA− symport mediated by AUX1/LAX influx carriers. Once inside the neutral cytosol, auxin is deprotonated and becomes trapped inside the cell. Auxin can leave the cell by auxin efflux carriers such as PIN-FORMED (PIN) proteins and P-glycoproteins (PGP) of the ATP-Binding Cassette family B (ABCB) transporter family. ABCB activity can be modulated by 1-naphthylphthalamic acid (NPA) and flavonoids that interfere with the interaction of ABCB and a protein that regulates it, TWISTED DWARF 1 (TWD1). The polar subcellular localization of PINs determines the direction of auxin flow out of the cell and thus the unidirectional auxin flow within tissues. Steffen anneste1, Jiří Friml, http://www.sciencedirect.com/www.cell.com
Furthermore, the transportation is more or less an active process. The long distance transportation appears to take place through sieve tubes and it is a facilitated mechanism. The rate of auxin movement is about 6.4-20 mm/hr, which is many times faster than the rate of diffusion. It is now clear that the transportation is through carriers. Auxin exists in ionized form called A (-) and uncharged form called AH. Transportation across the membranes is through carriers and in ioized form. But transportation in free space is diffusion and it is in AH form. One of the proteins that is responsible for efflux transport of auxins is PIN; there are several forms of these proteins.
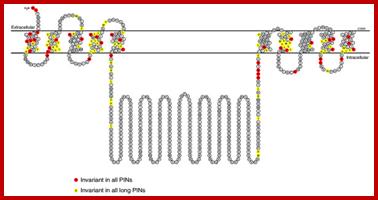
www.lhr.ueb.cas.cz

Below-The predicted structure of PIN proteins. The sequence shown is derived from AtPIN7; the positions marked in yellow are invariant in sequences of all 'long' PINs, the positions marked in red are invariant in sequences of all PINs. http://www.ncbi.nlm.nih.gov/

Transport across the cell involve ABC proteins, trends in plant science; IAA is transported across plasma membrane in protonated form, then it is transported across the cell to exoplasm with Ca2+ import and IAA export by an antiport mechanism; plant cell; www.cell.com
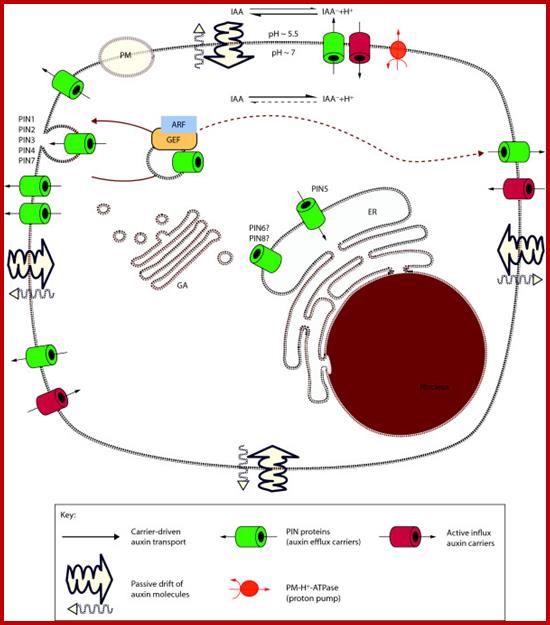
Schematic diagram of an idealized plant cell and the role of specific PIN proteins in auxin management at the cellular level. The low pH in the apoplast (the region outside the cell membrane comprising the plant cell wall) is maintained by the activity of the plasma membrane H+-ATPase. In the acidic environment of the apoplast, a relatively high proportion of auxin molecules stay protonated (un-ionized; indole-acetic acid (IAA)) and these can enter the cell directly via passive diffusion. In its ionized (dissociated) form (IAA- + H+), auxin cannot cross membranes by passive diffusion; it needs to be actively transported by carriers. Ionized auxin molecules can enter cells via active transport by auxin-influx carriers. In the relatively higher pH of the cytoplasm, auxin molecules undergo almost complete dissociation. The asymmetric positioning of the auxin-efflux carriers from the 'long' PIN subfamily at the plasma membrane then determines the direction of auxin efflux from the cell. Localization of AtPIN5 (from the 'short' PIN subfamily) at the membranes of the endoplasmic reticulum leads to compartmentalization of auxin into the lumen of the endoplasmic reticulum, where it undergoes metabolic conversion. PINs are efflux carriers of auxins; PM, plasma membrane; ER, endoplasmic reticulum; GA, Golgi apparatus. http://genomebiology.com/
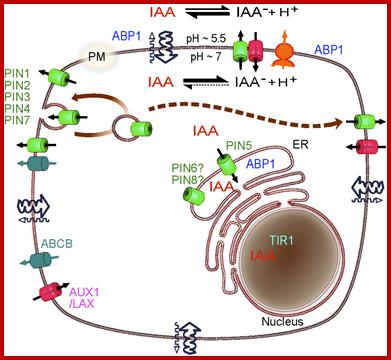
Schematic diagram of auxin fluxes and potential sites of auxin perception in the plant cell. The pH at the outer side of the PM is maintained at approximately 5.5 by the H+-ATPase activity of the PM (orange). As a consequence, a proportion of auxin (IAA) molecules in the apoplast remain protonated and can enter the cell via diffusion (wavy arrows). Auxin also enters cells through the action of specific uptake carriers of the AUX/LAX family (red). In the relatively higher pH of the cytoplasm, auxin molecules are deprotonated. The resulting ions cannot pass across the PM by diffusion and, instead, must be transported by efflux carriers including the PIN auxin transporters (green) and the MULTIDRUG RESISTANCE/P-GLYCOPROTEIN ABCB transporters (light blue). The coordinated polar localization of the auxin-efflux carriers from the long PIN subfamily at the PM determines the directionality of the auxin flow within the tissue. The long PIN proteins undergo constitutive endocytic recycling, which allows dynamic changes of PIN polarity by a transcytotic mechanism. PIN5, a member of the short PIN subfamily, is found at the membranes of the ER and leads to compartmentalization of auxin into the lumen of the ER. A number of auxin metabolic enzymes are also found in the lumen of the ER, and auxin metabolic profiling suggests that auxin entering the ER through PIN5-mediated transport rapidly undergoes metabolic conversion. The presumptive auxin receptor ABP1 (blue) is present both in the ER and in the apoplast. The receptor for the transcriptional auxin response pathway, TIR1 (light blue), is found in the nucleus. The different localizations of the long and short subfamilies of PIN efflux carriers, together with the spatial separation of the auxin receptors and localization of metabolic enzymes, implies that auxin signaling and metabolism, as well as auxin molecules themselves, are compartmentalized within the plant cell. Adapted from Křeček et al. (2009www.plantphysiol.org
Effects on general metabolism:
Isolated tissues, hypocotyls segments, epicotyl segments, leaves, excised roots and even whole plants have been used to monitor various biochemical and physiological responses to hormone treatment. Auxin has been found to accelerate cellular metabolism in treated tissues. Particularly respiratory rate increases by at least by 20%.
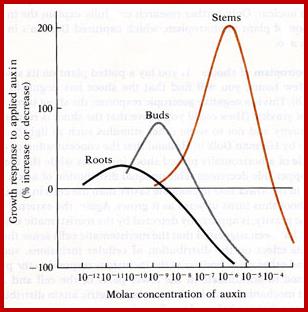
Response to concentrations of Auxins; saowalucktunpoomee.blogspot.com
The general activation of metabolic processes in terms of turnover is all pervading. Even mitochondria and plastids show increased activity. Many of the increased metabolic activities in response to auxin treatment have been attributed to the changes in membrane permeability and activation of some membrane factors. As a consequence of increased respiratory activities, photosynthetic activity, amino acid metabolism, nucleic acid synthesis and protein synthesis and others, cells build up the required materials for their growth.
Auxin Signalling:
Plant growth involves interaction between metabolites such as sugars, phytohormones and their action on gene expression. Auxin as a signaling molecule has various effects depending upon the tissue where it acts.
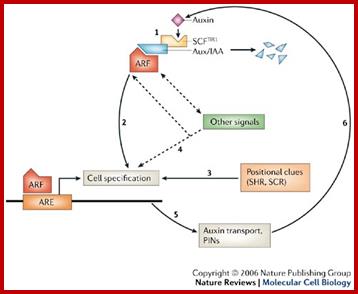
Auxin signalling and auxin transport in roots. William D. Teale, Ivan A. Paponov and Klaus Palmehttp://www.nature.com/
The Arabidopsis thaliana root tip is a commonly used model system in plants. Here, we describe the relationships between auxin signalling, auxin transport and cell specification. In step 1, auxin leads directly to the destabilization of Aux/IAA proteins, allowing auxin response factors (ARFs), which are transcription factors, to control the transcription of auxin-regulated genes (see also Fig. 1). In step 2, the subsequent expression of certain auxin-inducible genes, such as PLETHORA, initiates a complex chain of cell-specification events. However, as in step 3, cell specification is also dependent on self-generated positional cues, for example SHORTROOT (SHR) and SCARECROW (SCR), which mark the central cylinder and the endodermis/quiescent centre, respectively. In step 4, the presence of auxin is not sufficient for cell specification. For example, even though it is required for the first events in root specification, auxin alone cannot induce root formation. Therefore, interactions between this and other signalling pathways define the final cell specification. In step 5, the correct polarization of the auxin-transport machinery (the PIN proteins) is a result of as yet unknown polarized markers that are laid down by cell specification. In step 6, changes in auxin concentration that are a result of auxin transport control the expression of the early auxin-responsive Aux/IAA genes and the regulation of auxin-inducible transcription by ARF transcription factors. IAA, indole-3-acetic acid. William D. Teale, Ivan A. Paponov and Klaus Palme

www.imgarcade.com
The role of TIR1 in ubiquitination and regulated degradation of Aux/IAA transcription factors has been recognized for some years, but recent results have shown that TIR1 itself is also the binding site for auxin. The affinity and specificity of TIR1 match properties anticipated of a nuclear auxin receptor and we look at how they compare with the properties of ABP1. We also consider the mechanism of auxin action via TIR1 and the likelihood that the TIR1 family could account for all auxin responses. It seems likely that the TIR1 system can account for a large part of the repertoire of auxin-mediated responses, but maybe not all. http://www.sciencedirect.com/
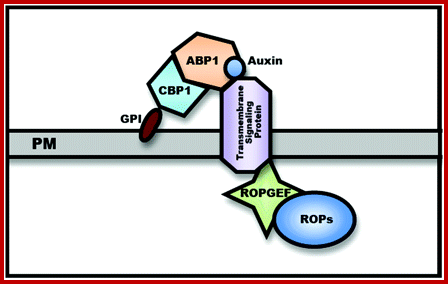
A Hypothetic Model for the ABP1-Based Cell–Surface Auxin Receptor Complex. An GPI-anchored protein (CBP1) is proposed to bind the C-terminus masking the C-terminal KDEL ER-retention signal and facilitating ABP1 secretion to the PM. CBP1 alone is unlikely to transmit auxin signal across the PM. A transmembrane protein is speculated to act as an auxin co-receptor with ABP1 to transmit the auxin signal to PM-localized RopGEFs that activate ROPs at the PM. http://mplant.oxfordjournals.org/
Cellular Auxin Signaling;
Auxin-binding protein 1 (ABP1) was discovered nearly 40 years ago and was shown to be essential for plant development and morphogenesis, but its mode of action remains unclear. Here, we report that the plasma membrane–localized transmembrane kinase (TMK) receptor–like kinases interact with ABP1 and transduce auxin signal to activate plasma membrane–associated ROPs [Rho-like guanosine triphosphatases (GTPase) from plants], leading to changes in the cytoskeleton and the shape of leaf pavement cells in Arabidopsis. The interaction between ABP1 and TMK at the cell surface is induced by auxin and requires ABP1 sensing of auxin. These findings show that TMK proteins and ABP1 form a cell surface auxin perception complex that activates ROP signaling pathways, regulating nontranscriptional cytoplasmic responses and associated fundamental processes. http://www.scoop.it/; www.sciencemag.org
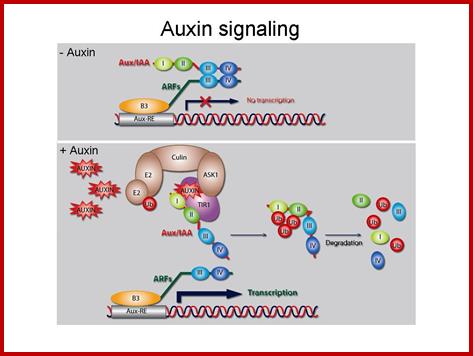
Auxin activating a specific Gene; http://pixgood.com/
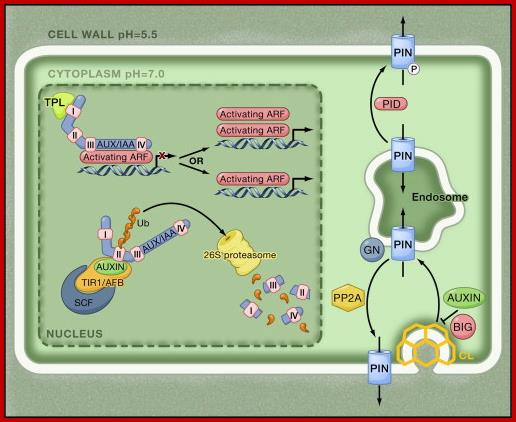
At low auxin concentrations, Aux/IAA transcriptional repressors are more stable and dimerize through their domains III and IV with auxin response factor (ARF) transcription factors. Through their binding to ARFs, Aux/IAA transcriptional repressors recruit the transcriptional corepressor TOPLESS (TPL) to activating ARFs, by which they are rendered transcriptionally inactive. At higher concentrations, auxin serves as molecular glue between domain II of Aux/IAA transcriptional repressors and TIR1/AFB F box proteins, thereby stimulating Aux/IAA ubiquitination by SCFTIR1/AFB E3 ligase and causing subsequent targeting for proteolysis mediated by the 26S proteasome. Degradation of Aux/IAAs derepresses the ARF activity on transcription. It is not clear whether ARFs act as monomers, dimers, or both. Outside the nucleus, PIN auxin efflux carriers cycle continuously between endosomal compartments and the plasma membrane. The exocytotic step requires the activity of GNOM, an ADP-ribosylation factor GTPase guanine nucleotide exchange factor (ARF-GEF), whereas endocytosis occurs in a clathrin-dependent manner. The PIN phosphorylation status, controlled by counterbalancing activities of PINOID kinase (PID) and protein phosphatase 2A (PP2A), affects the affinity for the apical or basal targeting pathways. Auxin inhibits PIN endocytosis through an unknown mechanism that requires BIG protein, the function of which is unclear. http://www.sciencedirect.com/
Effect on Nucleic acid synthesis:
Auxins show some dramatic effects on growth and development of plants. Though the immediate effect of auxin is known to be at the plasma membrane and cytosolic level, with time its effect on gene activation and protein synthesis is over bearing. The effectiveness of Auxin’s activity is due to the presence of auxin binding proteins. They act as receptors and after complexing with the auxins, they are rendered highly active.
The effect of auxins induced cell elongation does not require DNA synthesis, but in long term effect, DNA synthesis is always accompanied with cell division. The interaction between auxin and cytokinin has been interpreted as at the auxin plays permissive role in DNA synthesis and cytokinin stimulates it.
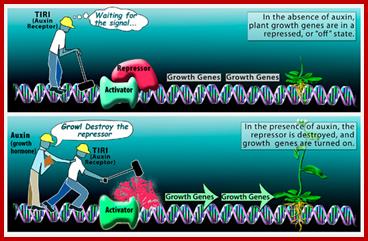
Scientist figure out plant growth mechanism; When a plant is not growing, auxin--a class of plant growth hormone--is not produced, and growth genes are repressed, or turned "off." But when conditions are favorable for growth, auxin is produced and binds to a molecule known as TIR1. The auxin-TIR1 complex in turn signals for the destruction of the repressor protein that keeps growth in check, and growth promoting genes are turned "on.". Auxin binding to its accessory protein leads to destruction of repressor and activation auxin response activator; www.nsf.gov
Auxins do not induce transcription immediately after application of the hormone. But auxin induced transcription sustained for 49-50 minutes. But auxin after effect on increased translational activity has been explained as due to the activation of translational machinery through the activation of translational factors. However, transcriptional activity in response to auxin at later stages results in the synthesis of all species of RNAs, such as mRNAs, tRNAs and rRNAs. Among the population of mRNAs induced by auxins, some specific mRNAs for cellulase, tubulin and others, are found is higher concentrations.
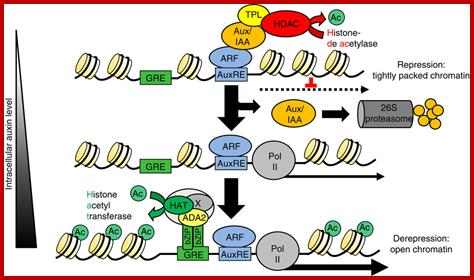
http://www.nature.com/
In higher plants, the hormone auxin orchestrates a diverse array of developmental and environmental responses mainly exerted via transcriptional control. In its absence, auxin-mediated transcription is postulated to be repressed by histone deacetylases, which convert chromatin into a highly packed inactive state. Here we present a converse mechanism where Arabidopsis bZIP11-related basic leucine zipper (bZIP) transcription factors interact via an amino-terminal activation domain with ADA2b adapter proteins to recruit the histone acetylation machinery to specific auxin-responsive genes. Gain, loss-of-function and pharmacological approaches as well as chromatin immunoprecipitation experiments addressing various components of the recruitment and acetylation machinery substantiate the proposed mechanism. Importantly, G-box-related cis-elements, frequently found in auxin-induced promoters, are shown to bind bZIP11-related bZIPs and to function as quantitative modulators of auxin-induced transcription. In conclusion, we describe a regulatory activation mechanism that serves as a rheostat to modulate auxin-mediated responses. Christoph Weiste & Wolfgang Dröge-Laser; http://www.nature.com/
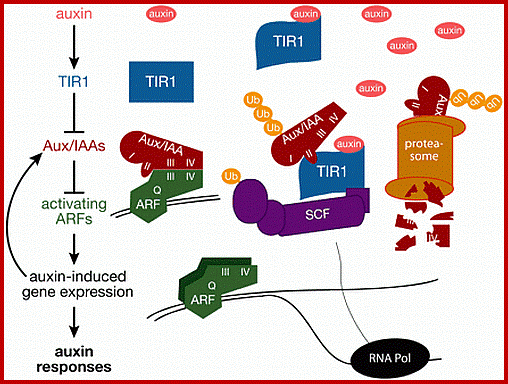
Once auxin is transported into cells, it binds to its receptor protein TIR, which inturn binds ARFs /IAA/Auxin protein complex which are acting as repressors. IAA-TIR binding leads to activation of SCF mediated degradation of IAA/Aux binding protein which frees ARFs and now ARFs bind to ARE promoter elements. Aux/IAA binding proteins were acting as repressors. The auxin-TIR1 now binds to Aux/IAA proteins and using SCF complex ubiquitinate AUX/IIA proteins and feed to proteosomes for degradation. Thus the ARFs become active and activate specific genes to which they are bound. This is general mechanism for auxin induced gene activation. www.clfs.umd.edu
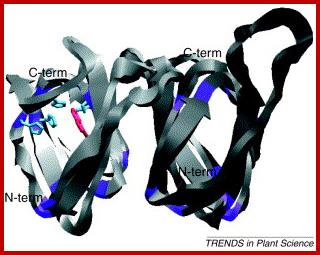
Auxin binding protein (ABP); www.cell.com
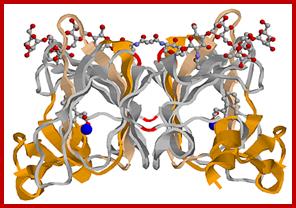
Auxin binding protein (ABP)-dimer
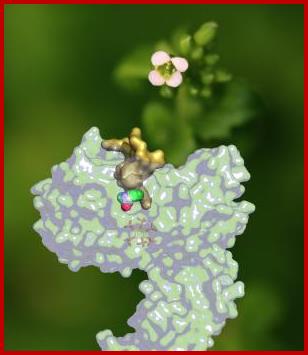
TIR1 protein bound to auxin activates Auxin response factors and thus gene expression. plantbiotechinfo.blogspot.com; www.sciencedaily.com
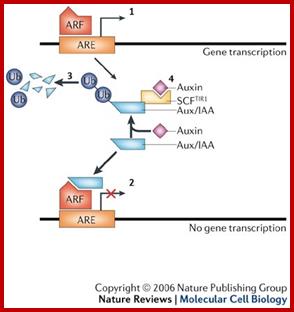
Auxin in action: signalling, transport and the control of plant growth and development; www.nature.com
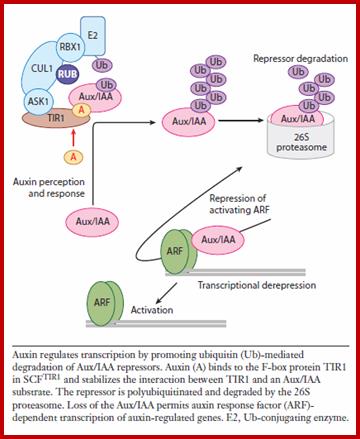
Our work has shown that auxin promotes the rapid degradation of a family of transcriptional repressors called the Aux/IAA proteins via the ubiquitin proteasome pathway. Auxin interacts directly with a ubiquitin protein ligase called SCF-TIR1, and promotes an interaction between the E3 and the Aux/IAAs. We are now studying the mechanism SCF-TIR1 action and the role of the complex in various aspects of plant growth. In addition, we are turning our attention to the complex transcriptional networks that mediate auxin growth responses. Our ultimate goal is to understand the systems that mediate auxin-dependent development at the level of the cell and organism.labs.biology.ucsd.edu
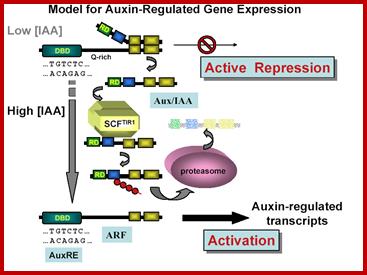
The above figure proposed by Thomas Guilfoyle provides the input how auxin mediated specific genes are activated. This proposal is considered to be the most famous among plant molecular biologists.
Thomas Guilfoyle; The Roles of Auxin Response Factor Domains in Auxin-Responsive Transcription; Shiv B. Tiwari, Gretchen Hagen and Tom Guilfoyle
We are studying how the plant hormone auxin regulates gene expression in plants. Currently, we are focusing on two classes of transcription factors which regulate expression of genes that respond within minutes to changes in auxin concentration. Members of one class of transcription factors, referred to as Auxin Response Factors (ARFs), bind to Auxin Response Elements (AuxREs) found in promoters of auxin-responsive genes. ARFs contain a novel DNA-binding domain that binds with specificity to TGTCTC AuxREs, a repression or activation domain, and a dimerization domain. A second class of transcription factors, referred to as Aux/IAA proteins, consist of very unstable nuclear proteins encoded by auxin-responsive genes. Aux/IAA proteins do not contain a DNA-binding domain, but do contain a repression domain and a dimerization domain related to the dimerization domain found in ARFs. Members of the ARF family can selectively dimerize with themselves and with members of the Aux/IAA family.
In plant cells with low amounts of auxin, ARF transcriptional activators are dimerized with Aux/IAA repressors on AuxREs of auxin-responsive genes. Under these conditions the genes are repressed and not transcribed or transcribed at low levels. When auxin concentrations increase, Aux/IAA repressors become less stable and are rapidly degraded by the ubiquitin-proteasome pathway, resulting in derepression/activation of the auxin-responsive genes. Gaining insight into the molecular mechanisms involved in the interplay of many different ARF and Aux/IAA proteins to regulate genes in response to auxin is the objective of current research in my laboratory.
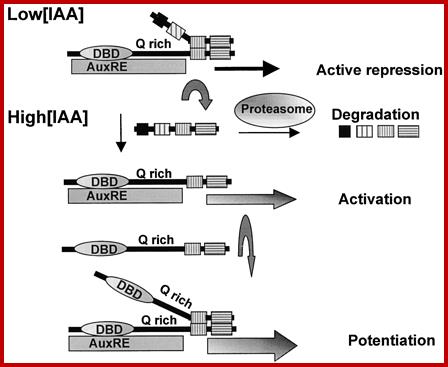 http://www.plantcell.org/
http://www.plantcell.org/
When auxin concentrations are low or below a threshold, early auxin response genes containing TGTCTC AuxREs are actively repressed, because Aux/IAA repressor proteins are dimerized to ARF transcriptional activators, preventing gene transcription. When auxin concentrations are increased, Aux/IAA proteins turn over more rapidly as a result of their being degraded more rapidly through the proteasome pathway (Rogg and Bartel, 2001; reviewed byKepinski and Leyser, 2002). This more rapid degradation of Aux/IAA proteins effectively relieves the repression of early auxin response genes, resulting in gene activation. Gene activation might be enhanced further by the dimerization of ARF transcriptional activators to ARFs that are bound to AuxRE target sites. In this model, the auxin-sensitive target is the CTD dimerization domain, and the ARF DNA binding domain and activation/repression domain function independently of auxin.
Auxin response factors (ARFs) are transcription factors that bind to TGTCTC auxin response elements in promoters of early auxin response genes. ARFs have a conserved N-terminal DNA binding domain (DBD) and in most cases a conserved C-terminal dimerization domain (CTD). The ARF CTD is related in amino acid sequence to motifs III and IV found in Aux/IAA proteins. Just C terminal to the DBD, ARFs contain a nonconserved region referred to as the middle region (MR), which has been proposed to function as a transcriptional repression or activation domain. Results with transfected protoplasts reported here show that ARFs with Q-rich MRs function as activators, whereas most, if not all other ARFs, function as repressors. ARF DBDs alone are sufficient to recruit ARFs to their DNA target sites, and auxin does not influence this recruitment. ARF MRs alone function as activation or repression domains when targeted to reporter genes via a yeast Gal4 DBD, and auxin does not influence the potency of activation or repression. ARF CTDs, along with a Q-rich MR, are required for an auxin response whether the MRs plus CTDs are recruited to a promoter by an ARF DBD or by a Gal4 DBD. The auxin response is mediated by the recruitment of Aux/IAA proteins to promoters that contain a DNA binding protein with a Q-rich MR and an attached CTD.
Effect on protein synthesis:
As mentioned earlier, auxin, in many systems enhances the rate of protein synthesis very early without the concomitant increase in RNA synthesis; which suggests that auxins earlier effect is on translational activity. This may be achieved either through the activation of inactive mRNPs, or through the activation of translational machinery and translational factors. Increase in polysome content in response to auxin treatment in many plant cells is good evidence in support of auxins’ promotive effect on protein synthesis. However, the increased level of protein synthesis at later stages is actually due to the auxin induced transcriptional activity, which really sustains the translational activity for a longer period of time. Though auxin enhances the synthesis of almost all house keeping enzymes, it also induces the synthesis of specific proteins like cellulase, cellulose synthase, tubulins and other specific factors required for cell elongation. It should be remembered that the specific effect of auxin on the expression of a particular protein product depends upon kind of tissue involved. Auxin induced activation of polymerization of tubulins into microtubules is another interesting feature of auxins effect.
Effect on cell elongation:
Among all the hormonal effects, the effect of auxin on cell elongation has been studied in detail. For a long time, the exact mechanism of auxin induced cell elongation has remained unsolved. For historical interests it is essential to understand a few theories which where proposed in the past to explain the mechanism. In this text recent views have also been provided.
Turgidity theory:
This is probably the oldest of the theories that have been proposed so far. This theory is based on the assumption, that auxin stimulates respiratory and other metabolic activities.
As a result, the osmotically inactive components of cells are degraded to osmotically active molecules. This in turn affects the diffusion pressure deficit of the cell and water potential gradient is created between the growing cells. So water from neighboring other cells, particularly xylem diffuses into the activated cell passively. As a consequence of this turgour pressure that builds up within the cell, the cell is stretched from within, thus the cell elongates. According to this concept the cell elongation is a passive phenomenon.
Auxin’s effect on cells elongation or expansion; http://www2.mcdaniel.edu/www.bio.miami.edu
It is true that auxins enhances the respiratory activity, but for the cell to elongate, due to the turgour pressure developed inside, the cell also requires loosening of the cell wall, without which whatever turgour pressure that develops, cannot force the cell to elongate or expand. In recent years, with the use of refined techniques, it has been demonstrated that in many plant systems the auxin induced cell elongation takes place under negative turgour pressure. Furthermore, respiratory inhibitors like DNP, cyanide inhibit auxin mediated cell elongation, which suggests that cell elongation is an active process. Because of these the turgour theory is not favored. One of the proteins involved in cell expansion is expansin that loosens the cell wall fibers.
Gene activation theory:
With new discoveries in the field of molecular biology, plant scientists also thought, that auxin brings about the cell elongation through gene activation. Bonneret.al. suggested that as auxins are acidic in nature, they can easily bind to basic proteins found associated with the chromatin material. The binding of auxins to histones, certain segments of DNA’s are freed from the surrounding histone proteins for transcriptional activity. As a result, the required mRNAs for cellulase are produced. As cellulase is required for degradation and loosening of the cell wall, they proposed gene activation. The combined effect of gene activity and auxin induced turgour pressure has believed to be mechanism of cell elongation. Further elongation stops at later stages and the synthesis of cell wall materials continues which again is due to the activation of gene expression.
This theory however falls short of its expectations because transcriptional activity in response to auxin increases only after 15-30 minutes later, but the apparent growth of cell starts as early as 10-15 minutes after auxin treatment. So this theory also fails to explain the early phase of growth, moreover, the binding of auxin to the histone in bringing about gene expression is no more tenable.
Acid theory:
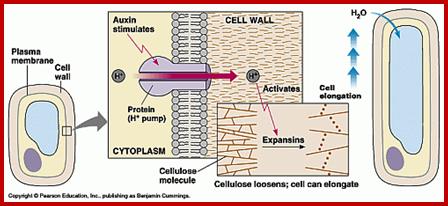
Cleland and others have demonstrated that stem segments on exposure to auxin, secrete protons into external medium and render it acidic. Sharp fall in pH of the medium due to auxin treatment has been demonstrated by many workers. Proton secretion has been attributed to the activation of H/ATPase found in the plasma membrane. As the protons diffuse through the cell wall, the pH in that region falls and acidic form cellulase will be activated. As a result, the cell wall fibrils are cut and loosened which greatly facilitates the elongation of cells.
Acid theory explains the early effect of auxin on cell elongation even before
the auxin induced transcription starts. This theory also assumes that the internal pressure responsible for the elongation as turgour pressure. It is now known that not in all cases turgour pressure has been demonstrated as the cause for cell elongation. However, this theory explains the early effect of auxin on cell elongation.
Plant cells elongate irreversibly only when load-bearing bonds in the walls are cleaved. Auxin causes the elongation of stem and coleoptile cells by promoting wall loosening via cleavage of these bonds. . About 20 years ago, it was suggested that the wall-loosening factor is hydrogen ions. This idea and subsequent supporting data gave rise to the Acid Growth Theory, which states that when exposed to auxin, susceptible cells excrete protons into the wall (apoplast) at an enhanced rate, resulting in a decrease in apoplastic pH. The lowered wall pH then activates wall-loosening processes, the precise nature of which is unknown. Because exogenous acid causes a transient (1-4 h) increase in growth rate, auxin must also mediate events in addition to wall acidification for growth to continue for an extended period of time. These events may include osmoregulation, cell wall synthesis, and maintenance of the capacity of walls to undergo acid-induced wall loosening. At present, we do not know if these phenomena are tightly coupled to wall acidification or if they are the products of multiple independent signal transduction pathways. Rayle DL, Cleland RE
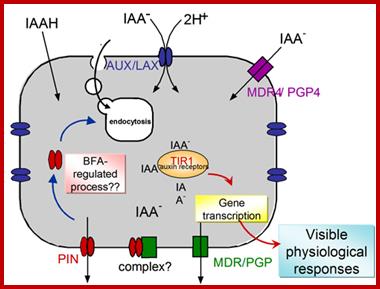
IAA enters cells passively or in protonated form; http://www.ncbi.nlm.nih.gov/
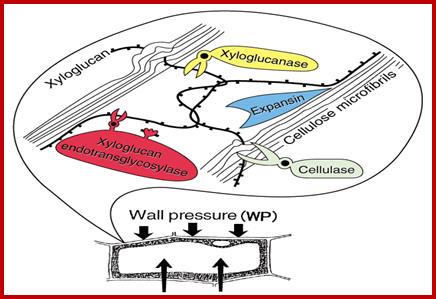
Cell expansion requires the role of Microfilaments and microtubules. When hypocotyls are treated with IBA within 30 minutes, one observe increase in Microtubules and microfilaments as deduced by mRNA isolated from treated tissues and in vitro translation studies show increased synthesis of MTs and MFs (grkraj). Auxin also induces cellulase enzymes which act on cellulose fibers and loosen the cell wall and facilitate the expansion due to increase in turgid pressure. www.glycoforum.gr.jp
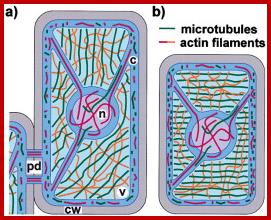
The Interphase Plant Cytoskeleton’
Cytoskeletal structures in nongrowing cells (a) and in cells that elongate by diffuse growth (b). Unless stimulated by wounding or pathogen attack, fully expanded cells such as the one depicted in (a) are often free of cytoplasmic strands and contain nuclei located in the cell cortex. Cytoplasmic regions are indicated in dark blue, and in light blue when shown underlying the vacuole, or in purple when shown overlaying the nucleus. pd, plasmodesmata; c, cytoplasm; n, nucleus; v, vacuole; and cw, cell wall. www.cell.com
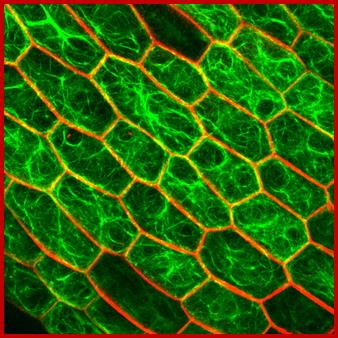
Cytoskeletal elements cuase cell elongation;www.slcu.cam.ac.uk
Cytoskeleton theory:
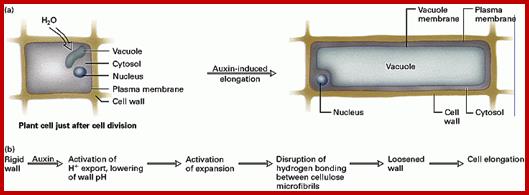
This theory is the most recent theory and it is based on many experimental evidences. It is very important to know that auxin induced cell elongation or growth exhibits two phases. The first phase is initiated as early as 5-15 minutes after treatment. It is rapid and lasts for 30-45 minutes. This phase is insensitive to the inhibitors of transcription and translation. But it is highly sensitive to colchicine treatment. The above features suggest that the early rapid growth does not require transcription or translation products. But it requires microtubule formation because colchicine inhibits polymerization of tubulin monomers into microtubules. On the other hand, the second phase of growth is slow but steady. It is sensitive to Actinomycin-D, CHI and also colchicine. It means the second phase requires transcriptional products, translational products and polymerization of tubulins into microtubules which act as cytoskeletons.
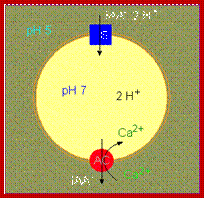
In the first phase, auxin first enhances the rate of respiration, thereby ATP production increases. At the same time, it also activates nucleating centers and H/ATPase pump located in the plasma membrane. Nucleating centers are a group of aggregated tubulin monomers which on activation start assembling available tubulin monomers from the cytosolic pool. Thereby, a large number of microtubules grow and elongate from the nucleating centers. Polymerization of tubules requires GTP and ATPs as energy source. The hydrolysis of them generates significant amount of H+. Meanwhile, the activated H/ATPase pumps excrete H+ into the exterior of the plasma membrane by active process. As the pH in the cell was becomes acidic, the acidic form of cellulase enzyme which are already present become active glucanase activity they hydrolyze the cellulose fibrils, thus the cell walls fibrils loosened. In this process, certain amount of Ca2+ ions are also removed from the middle wall, which renders the middle wall more labile and plastic. As more and more microtubules grow and elongate and build up, they build up a kind of mechanical force within the cell and the cell is stretched. MTs contribute to the deposition of cell wall materials to be transported and deposited out side the plasma membranes. The elongation is further facilitated by the loosened cell wall. Thus the cells grow in length.
The second phase of cell elongation however required more and more of tubulin pool because most of it is depleted in the first phase. So it also requires fresh synthesis of tubulins, cellulase enzymes and other required factors for the sustained cell elongation as per the demand transcriptional activity increases and the RNAs produced are used by the translational machinery and more of proteins are synthesized. Though there is an increase in the synthesis of most of the house keeping proteins, the increased synthesis of tubulin, cellulase and cellulose synthesizing enzymes is significant. Utilizing tubulin monomers continue to polymerize and more of microtubules generate at the same time loosening of cell wall continues. There by the second phase of cell elongation proceeds slowly but steadily. That is why the second phase is so sensitive to the inhibitory actions of actinomycin D, CHI and colchicine.
www.thedollblog.com
This theory appears to explain most of the observed properties of cell elongation. Furthermore, it also explains how cells elongate even in the absence of sufficient turgour pressure within the cell.
Effect on new root formation:
Roots developing from any part of the plant body other than the radicle are called adventitious roots. It is not an uncommon phenomenon to see the plant parts are propagated by inducing new root formation. But the exogenous supply of auxins to stem and leaf cuttings readily induces the new root formation, which ensures vegetative propagation. The synthetic hormones like IBA and NAA are more effective in new root formation than the native IAA.
If defoliated stem cuttings are continuously washed in water to leach out the endogenous auxins, they do not produce any adventitious roots in a nutrient media. But if such washed segments are treated Indole Butyric Acid for about 10-20 minutes and placed in a nutrient medium, stem cuttings produce a large number of roots. These roots normally develop from the terminally differentiated pericyclic tissue found around phloem tissue. Within 36-72 hours after treatment some of the founder cells in the said pericyclic region undergo transformation and organize into root primordia, which later grow through the cortex and emerge out of the stem.
www.driverlayer.com
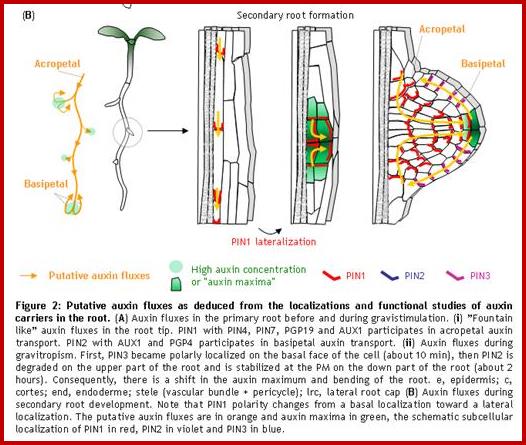
www.imgarcade.com
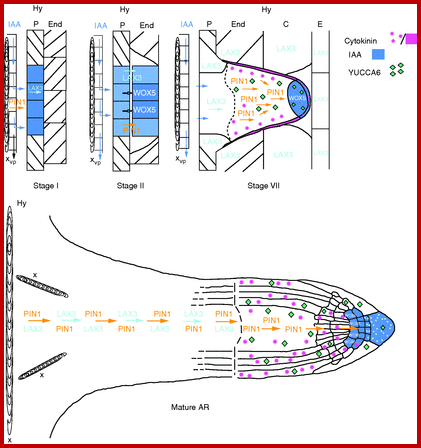
Model of auxin flow, gene expression and cytokinin localization during AR formation in planta. At stage I, auxin (IAA) is diverted from the basipetal flow along the vascular parenchyma cells (vp) adjacent to the protoxylem (x) of the hypocotyl (Hy) towards the pericycle (P) cells by PIN1, activating LAX3 and auxin accumulation (blue colour) in the founder cells. At stage II, auxin is maintained in the first-formed inner and outer AR layers by PIN1 (yellow arrow) and LAX3 (light-blue arrows), andWOX5 is expressed. At stage VII, PIN1 drives auxin flow towards the ARP tip throughout the middle cell files, because cytokinin (pink colour) downregulates PIN1 in the peripheral ARP layers. Cytokinin also downregulates LAX3, limiting the carrier activity at the ARP base (up to the dotted line). The auxin flow driven by PIN1 towards the tip results in an apical auxin maximum, limiting WOX5 expression at the distal tip, and here establishing the position of the QC. Auxin biosynthesis by YUCCA6 (green diamonds) contributes to auxin maximum positioning in the tip. LAX3 is also active in the Hy endodermis (End), cortex (C) and epidermis (E) around the ARP, possibly favouring protrusion. In the mature AR, the auxin maximum (blue colour) encompasses the QC, flanking initials and cap cells (columella, in particular), and WOX5 QC expression is maintained. Auxin biosynthesis by YUCCA6 is also maintained (green diamonds), contributing to the persistence of apical auxin accumulation. Also cytokinin is present at the AR tip (pink stars), contributing to the maintenance of auxin homeostasis by a downregulation of PIN1 in the forming epidermis/cortex, and of LAX3 in the entire tip, except the cap (light-blue dots).PIN1 and LAX3 are expressed in the AR vasculature, and LAX3 expression stops at the elongation zone border (dotted line). http://aob.oxfordjournals.org/
The transformation and organization of pericyclic cells into root primordia is a phenomenon of dedifferentiation. Studies on molecular aspects of IBA induced new root formation in the hypocotyls of phaseolus vulgaris reveal that the new root formation is due to the differential gene expression. Using techniques like translation of isolated mRNAs from treated and untreated segments at different time periods in a cell free system and the analysis of invitro and in vivo protein products by polyacrylamide SDS gel electrophoresis and autoradiography and immunoprecipitation of labeled proteins by using specific antibodies, show that among the many proteins synthesized, the synthesis of 55-58 KD proteins and 105 kd proteins in the IBA treated segments increases significantly. The 55-58% proteins have been identified as α and β tubulin, which are the precursor for microtubules. Involvement of microtubular assembly and orientation during the differentiation and organization of pericyclic cells into root primordia is confirmed by the use of colchicine and cytochalasin B. The said drugs prevents the root initiation in the IBA treated hypocotyl segments within 36 hours after hormonal treatment, but the drugs have no effect if the hypocotyls that have already passed through 36 hours after IBA treatment. The above results indicate how auxins can bring about new root formation by inducing differential gene expression.

IAA concentration against organ development
Some of the concepts of auxin action on gene expression has been shown below in the form consolidated figures which are self-explaining.
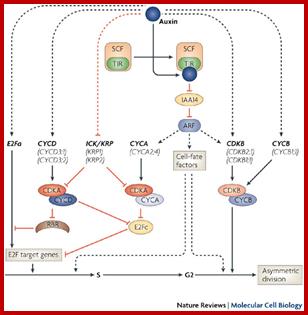
Lateral root develop from preexisting roots and new roots develop from stems. The root initials start from cambial founder cells. It is important to remember that even plant tissue, as in the case of animal systems, contain stem cells, called them as founder cells. Proper signals can induce organ formation from such stem cells. Auxin activates founder cells in pericycle to enter into G1-S transition. It is now clear that auxin induces certain cyclins and CDKs (B) types. Auxin induced transcriptional mechanism perhaps go through activators, Auxin binds to auxin response factors TIR1, which in turn translocates into the nucleus and bind to auxin response elements of genes. However the degradation ARF/IAA/Auxin protein complex by SCF –TIR is important for the ARFs to bind to auxin response elements properly. It is also possible several other factors are involved in induction of lateral roots; probably auxin binding protein (ABP) is one among them.
Effect on apical dominance:
Plants like pines and other conifers exhibit a growth pattern, which is quite distinct from a banyan tree or a tamarind tree. The conical growth of the pine plant is due to the predominant growth activity of the apical meristems where the growths of lateral buds are more or less suppressed. On the other hand, the growth pattern in banyan tree is diffused, where lateral branches grow as vigorously as the apical branches. The suppressive effect of apical buds on the growth of lateral buds is often called apical dominance. Such a phenomenon is not just restricted to only conifers, but it is also found in other plants.
Apical dominance has been explained as due to the action of auxin present in the main apex. This view is amply supported by an experiment, where if the stem tip is cut off, the axillary buds found below sprout immediately. Instead, if an agar block containing auxin placed over the decapitated stem, the axillary buds remain suppressed. It is clear from the above experiment, that auxin present in main apex some how inhibits the growth of the axillary bud. The severity of apical dominance is greater on the axillary buds present nearer to the main apex. Nevertheless, apical dominance exerted by the auxins can be overcome by the application of cytokinins to axillary buds. This is because cytokinin induces cytokinesis. Probably, the mitotic block that is operating in axillary buds may be due to the inadequate supply of cytokinins. But how the supply of cytokinin to axillary buds is made inadequate by the apical bud is not clear.
www.wps.prenhall.com
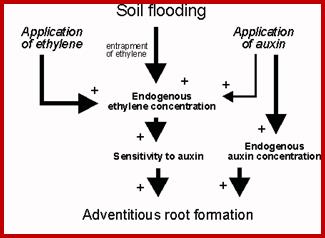
The idea of nutritional inadequacy to the axillary buds due to the stronger influence of apical meristems has been considered by many scientists. It is assumed that because of apical dominance, most of the nutrition is drawn towards the apex than to the axillary bud. This explanation for apical dominance has no conclusive evidences to prove the claim. On the other hand, studies have shown that higher concentrative of IAA induces the synthesis of ethylene. As apical buds contain more of auxins, it may induce the synthesis more of ethylene which in turn may inhibit the normal growth of the axillary bud. But by removing the apical bud, the concentration of auxin drops, so also ethylene hence axillary buds sprout.
Effect on Phototropism:
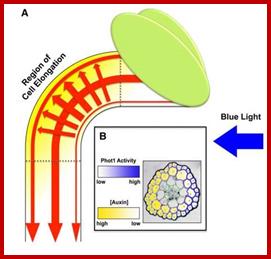
www.plantphys.info
www.plantphys.info
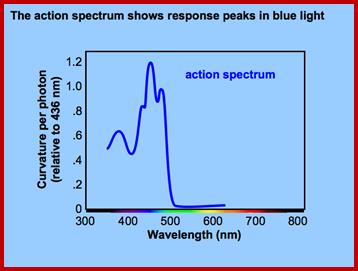
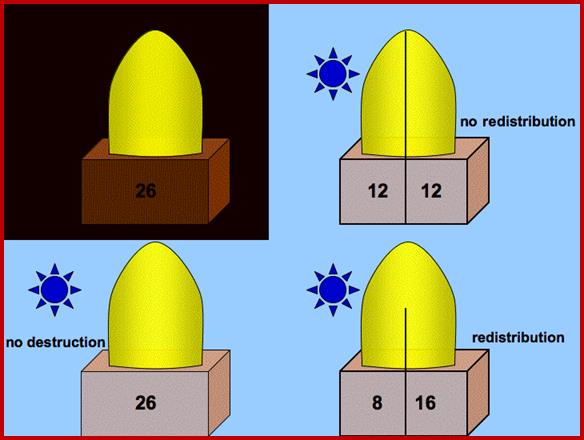
The growth curvature in stem apex in response to light is called phototropic movement. Such growth curvature in shoot tips can be induced even in the absence of light by placing auxin containing agar blocks asymmetrically on decapitated stem tips. This indicates that unequal concentration of auxin is responsible for unequal growth there by the curvature. But in light induced curvatures, how does light brings about unequal concentration of auxin and which part of the light spectrum is responsible for the curvature are the few questions that needs explanation. Using monochromatic light, it has been determined that the most effective spectrum is 445 mm. But the answer to the first question is still elusive and various theories have been proposed from time to time to explain this phenomenon.

Lateral Transport Theory:
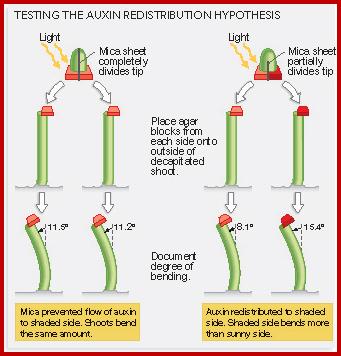
This theory was proposed by Cholodney & F.W. Went. Accordingly, the concentration of auxin in the stem apex is uniform all-round. Once the light rays fall on the stem from one side, it induces the movement of auxin from the illuminated region towards the darker region. By such movement, auxins accumulate in greater amounts in the darker region. The motive force for the movement of the auxin in response to light is attributed to the differential electrical charges on the said surfaces. Then he tips were illuminated from one side and the basipetally diffused auxin was collected and the amount of auxin found in the agar blocks was determined. According to the authors in the control tips they detected more of auxin in the far side the surface that was in the dark than from the surface that was illuminated. But in the tip with a mica plate the amount of auxin found in both the blocks was same. So it was deduced that the difference in the amount of the auxin detected in the blocks on the darker side is actually was due to lateral movement. Accordingly resultant differential concentration is responsible for differential growth, hence the phototropic curvature. The growth is dependent auxin concentration.

www.Plantphysiol.info
Recombinant GUS gene expression of a gene that is responsive to auxin shows the distribution of Auxin in response to light
Their claim was disputed by other workers, who used radioactive C-labeled auxin for exact quantitative determination of the transported auxin from one region to another. They did not find any substantial lateral transportation. So the lateral movement theory has remained unconvinced.
Inhibition of basipetal Transport Theory:
Gordon and others proposed that the unequal distribution of auxins in response to light treatment is not due to lateral movement, but due to the inhibition of basipetal movement from the illuminated side, which causes unequal concentration of auxins and growth curvature is due to it. Slow growth of the stems in day times and steady growth of the plants in intense sun light has been attributed to this effect. However this theory has not been tested with covincing experiments.
Photo inactivation Theory:
When blue light at 445 nm has been detected as the action spectrum for the induction of phototropic curvature, plant physiologists started looking for pigments that absorb blue light at 445 nm. This logic was based on the fact that known photo responses like photosynthesis and photoperiodic responses are due to specific pigments. The search for such pigments revealed the presence of B carotenes and riboflavin in the stem tips. As some plants, which respond to light induced phototropic movement, did not possess B carotenes it was deduced that riboflavin as the causative pigment. The photo inactivation was explained on the assumption that riboflavin after absorbing light gets activated and the same inactivates the auxin directly or destroys the auxin through certain IAA oxidase. This result in unequal concentration of auxin in the stem tip which in turn is believed to be responsible for the phototropic curvature again this theory has never been proved unequivocally.
Present concept:
In the past, many experimental results were based on very crude extraction methods. Such experimental results are not very convincing today. Use of radioactive isotopes i.e. (14C) IAA, solvent extraction methods combined with GLC analysis for qualitative and quantitative estimations of auxins have cast grave doubts about previous theories. In the light of recent work, it has been suggested that light has profound effect on inducing the synthesis or releasing the growth inhibitors like ABA. The release of ABA in the region where it is illuminated causes inhibition of growth in the said region. The effect of ABA on osmotic changes by creating effluxes of ions is well known. Moreover, it is now known that auxins could be made inactive or active by auxin binding proteins. If binding protein complexes with IAA, it is rendered physiologically active, if it is free, it remains inactive. So light induced changes in the concentration of Auxins and other Auxin binding factors are believed to bring about variations in the endogenous levels of active auxins. Such changes are ultimately responsible for phototropic growth curvatures,
For a long time the above concepts were accepted as facts, however, finding of phototropin, a blue light absorbing protein, has role in phototropic curvature is also an accepted fact. Phototropin exists in two forms; both are flavin binding proteins of mol.wt 110 Kda and 124Kda. Interestingly these proteins contain at least 11 kinase (PAS) domains; these domains are celled LOVE domains (Relation to Light, Oxygen and Voltage). They also contain different domains such as BTB and D1-to D15 domains. Phototropins act as light receptors and absorption leads to autophosphorylation at serine/threonine sites and they are involved in phosphorylation of many other membrane bound proteins. Membrane bound proteins have many roles in transportation of ions, transportation of Auxin/auxin receptor.
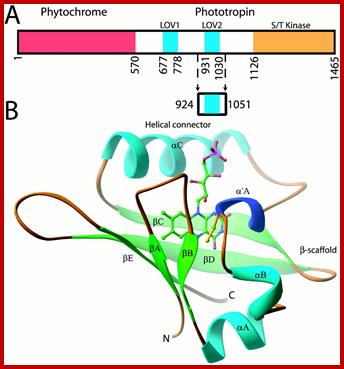

Adiantum phy3 domain and LOV2 structures. (A) Adiantum phy3 domain structure showing the N-terminal phytochrome chromophore domain bound to a phototropin.
Residues forming the LOV2 construct are marked by arrows. (B) RIBBON diagram of the phy3 LOV2 structure. The FMN cofactor is shown in the chromophore-binding pocket of LOV2 and is colored by elements: carbon, green; nitrogen, blue; oxygen, red; phosphorus, pink. C966 is at the N terminus of α′A and is colored yellow; http://www.pnas.org/
Phototropins1 and 2 versatile blue light abosbing light receptorhttp://www.cell.com/
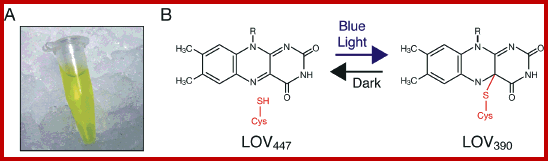
LOV-domain photochemical reactivity. (A) Purified preparation of bacterially expressed LOV2. (B) Schematic representation of LOV-domain photochemistry. Details of the reaction are described in the text. http://www.photobiology.info/
However the exact mechanism by which they bring about photrophic curvature movement is yet to be discerned.
Conformational changes of Love domain proteins called Phototropin in the presence of light and in the absence of light.
Plants regulate their growth directions in response to light
direction, and its response is called phototropism. Phototropism is a model
research to understand the regulatory mechanisms of auxin metabolisms in
response to environmental stimuli, and we are studying on this topic by a
molecular genetic approach using Arabidopsis mutants. We identified a signal
transducer RPT2 and a novel blue-light photoreceptor phot2, both of which are
required for the phototropic responses of Arabidopsis. We indicated that phot1
and phot2 show partially overlapping functions in two different responses, phototropic
response and chloroplast relocation, in a fluence rate-dependent manner, as
blue light receptors. To reveal the phototropin-signaling pathways, We studied
on the regulation of cytosolic Ca2+ concentrations by phototropins,
involvements of RPT2 and another phototropin-signaling factor NPH3 in
chloroplast relocation and stomatal opening, and functional sharing between
phototropins and other blue-light receptors cryptochromes. Now, study is
conducted on a dephosphorylation mechanism of NPH3 and transcriptional and
post-transcriptional regulations of RPT2 in response to blue light irradiation.
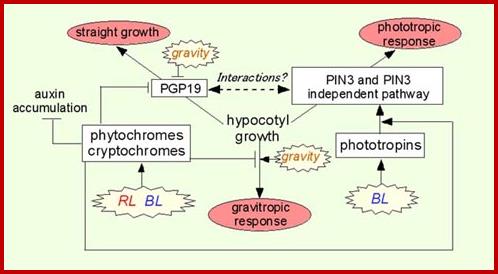
In addition, we revealed that photoreceptors phytochromes and cryptochromes
regulate auxin biosynthesis, metabolism, and transport in response to light
irradiation and indicated that a suppression mechanism of expression of auxin
transporter PGP19 by phytochromes and cryptochromes is one of mechanisms to
enhance the phototropic response by phytochrome and cryptochromes. Other
analyses on the light signaling and auxin mechanisms were also published by
collaborations with other research groups.
Light activates three kinds of photoreceptor families,
phototropins, phytochromes, and cryptochromes, and affects the plant growth
directions through changes of auxin biosynthesis/metabolism and transport. www.lookfordiagnosis.com
Effect on Growth Curvatures:
Growth movement of any part or the organ of the plant body in response to gravitational pull of the earth is referred to as geotropic movements. The most remarkable feature of the plant body is that, though both shoot and root system are derived from the same embryonic cells, they respond differently to the same gravitational stimulus. This property may be due to unique embryonic developmental programmes dictated by the inbuilt genetic factors, which probably enforce the respective organism to behave differently to different environmental factors.
Growth curvature away from the earth’s gravity is called negative geotropism and the growth of the roots towards earth is called positive geotropism. Most of the roots, with the exception of pneumatophores and coralloid roots which are negatively geotropic, exhibit positive geotropism. However, not all stem show absolute negative geotropism. It is a common observation that some of the underground stems like rhizomes, suckers, etc, grow obliquely in the soil and such growth movements are called dia-geotropic movements.
Mechanism of geotropic for with Curvatures:
Again, Cholodney and went are the pioneers in explaining geotropic movements. They proposed that stem apexes and root apexes require different concentration of auxins to bring about the maximal growth. In the sense, it is said, that the concentration of auxin that is favorable for the growth of shoot apex is inhibitory to the growth of the root apex. On the contrary, the concentration, that is optimal for the growth of the root apexes, is not adequate for the growth of the stem tips. It implies that stem cells require greater amount of auxins for the in maximal growth and root cells require very low concentration of auxins for their optimal growth. Based on these premises the mechanisms of geotropic responses have been explained.
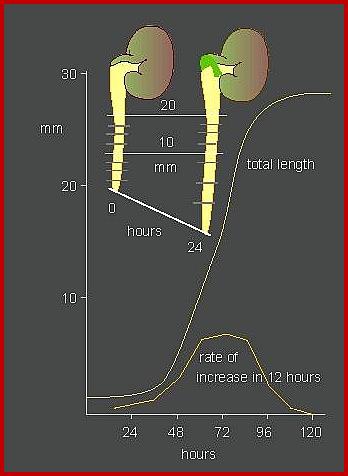
If a straight seedling is placed horizontally on the soil, shoots curl upwards and roots bend towards the soil. This behavior has been attributed to the gravitational force that acts upon the respective organs for they are endowed with different programmes, though both have the same genetic properties. Cholodney and went assumed that the gravitation once acts on both root and shoot. So auxins having certain mass of their own move downwards and accumulate in greater amounts in the lower cells which are in contact with the soil.
As higher concentration of auxin is promotive in stem tips the cells grow faster than the others. So the stem curves and grows upwards. On the contrary, as the higher concentration of auxin is inhibitory for the root cells, the growth of root cells is inhibited, but the cells containing less amount of auxins show maximal growth activity. Hence root tips curve towards soil and grow forwards in to it. Thus stems exhibit negative geotropic growth movements and roots show positive geotropic movements.
The above said theory enjoyed the general acceptance for a long period of time. But people realized that gravitational force, by its mass action, has greater effect on amyloplasts than on auxins found in the plant cells. Because of their greater mass, amyloplasts settle down on the plasma membranes of the cells as shown in the figure. The contact of the amyloplasts with plasma membranes acts as on irritant as a result growth of cells on that side of the membrane is inhibited but the growth on he other side is favored. But this explanation has never been favored because some plants which are lacking in amyloplasts also exhibit geotropic responses.
In recent years, research work on geotropic movements has revealed that the site of geotropic perception is not the root apex, and it requires root cap for its response. If the root cap is cut off, the decapitated root dies not show any geotropic responses. When the cap is replaced he roots respond normally for geotropic stimulus. This observation has been explained as due to the synthesis of ABA in the root caps. The ABA is transported basipetally, and then it also moves downwards due to the gravitational force. As the lower cells receive ABA their growth is inhibited. But the upper cells grow normally and bring about geotropic curvature.
Effect on Parthenocarpy:
Development of fruits without fertilization is known as parthenogenesis and the fruit is said to be parthenocarpic fruit. Auxins have been found to be effective in inducing parthenocarpic fruits in some plants. It has been demonstrated that the extracts of pollen grains also induce the development of parthenocarpic fruits, discharge of pollen tube contents into embryo sac is believed to be cause for the increase in he content of auxin in embryo sac. Some botanists suspect that pollen tubes carry some enzymes which produce more auxins.

It is important to note the synthesis of more auxins not lead to fertilization. Whether the act of fertilization has any stimulatory effect on the synthesis of auxin is not clear nonetheless many plants respond to auxin treatment produce parthenocarpic fruits.
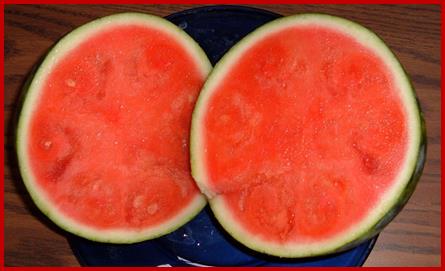
Induction of parthenocarpic fruits by auxins has greater application value in cultivating fruit yielding plants. The auxin induced fruits, besides seedless, they are larger in size and sweeter. Commercial production of such fruits brings more income to farmers.
Effect on Abscission
It is common to observe that older leaves, debladed petioles, abortive flowers, and often fruits, fall off from the plants. In the above said cases a distinct and characteristic layer of cells develop at the base of the petiole of the leaf or the pedicel of fruit, which acts as a weak point, hence the said structures fall down. The layer that is responsible for this process is called abscission layer or abscission zone.
Structure of Abscission Layers
It consists of a number of layers of thin walled cells which are rich in cytoplasm and actively dividing. Disappearance of middle lamella from the cells of this layer is a characteristic feature. This is due to the activity of pectinase enzyme. As a result, this part of the stalk becomes weak and the leaf or the fruit falls down by its own weight.
In many plants, just below the abscission zone towards the stem, a layer of actively dividing cells develops. This layer is called protective layer. This layer develops after the fall of leaf at the free surface, but in some cases the protective layers develop even before the fall of the leaves.
These cells by repeated cell divisions produce many layers of cells, which get suberized and protect the inner layer of cells from injury or infection. Thus, this layer acts as wound healing tissue.
Development of Abscission Layer:
Leaves fall with the age and fruits fall down after ripening, but in deciduous plants, onset of winter acts as the signal for the plants to shed their leaves. Sometimes, unseasoned cold waves induce premature falling of fruits. In all these cases, the falling of leaves or falling of fruits is due to the formation of abscission layer at the base of their stalks. The factors that induce the formation of abscission layers vary. In some cases, ageing acts as an important causative factor, in others environmental conditions like winter may act as the factor. In the case of aging the accumulation of senescing factors induce abscission layer formation. One of the senescing factors is believed to be ABA. Reduction in the quantity of auxin n the distal parts of the leaf is also known to be another causative factor. In the initial stages of abscission layer formation, if IAA is applied to distal part of the leaf, the development of the abscission layer is inhibited. Instead, if auxin is applied at a later stage of abscission the process I shortened and the leaf falls quickly.
Once, it was thought that ABA is mainly responsible for leaf abscission, but recent investigations indicate that ethylene is highly effective in inducing abscission layer formation, but the role of ABA is not totally ruled out. Incorporation of radioactive label during the abscission layer formation suggests, differential gene expression in the abscission zone results in the production of pectinases and cellulases, which by their activity breakdown the middle wall and also some cellulose. At the same time the cells in this zone become meristematic.
Uses:
The application of auxin to leaves and fruits is now known to prevent the development of abscission layer. Many synthetic hormones like NAA, IBA, 2,4-D have been found to very effective in preventing the premature fall of fruits, particularly commercial crops like citrus, apple, oranges, mangoes, grapes. The use of such hormones not only prevents the premature falling of fruits, but also increases the quantum of fruit setting. And fruits thus produced are larger and sweeter. Thus auxins can be used for commercial gains in the field of pomiculture.
Auxin induced new root formation:
Application of IAA to stem cutting ends induces new root formation and facilitates lateral root development. In fact application cooking Asafetida (an indole compound) also induces new root formation.
Initiation takes around 36 hrs, first, in the case of cuttings oh hypocotyls of soybeans, cells below pericycle layer and in between protoxylem elements of two exarch xylem elements get stimulated and they become large and the nucleolus increases to great extent, almost 2/3s of the nucleus; the nucleus itself gets enlarged almost 2/3ds of the cell, which indicates enhanced transcriptional activity. Also one can observe increased synthesis of Tubulin dimers and actin. In addition one of the ‘maps’ also appears in its proteins only around 36hrs after treatment, then by 48 hrs it disappears; it indicates its regulatory role. These results were observed using protein and mRNA analysis and precipitating using IgG against tubulins.
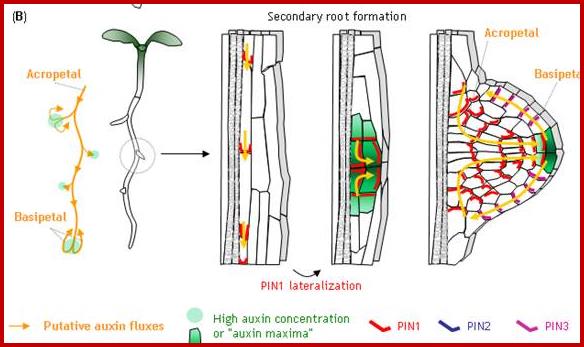
Plant Cell Biology.www.mol-biol4masters.org
Peptide-Mediated Regulation of Root Development;

Recent advances in peptide-mediated regulation of root development. CLE, RGF/GLV/CLEL, IDA, and CEP peptides are involved in several aspects of root development including lateral root formation (A), (B), (D), (E), protoxylem development (F), stem cell maintenance (G), and gravitropic response(C). Known pathways discussed in this review are shown near their approximate location in the root. Peptides are indicated in green text, receptors in blue. Upstream processes are yellow and downstream processes are red. Developmental output is indicated by black text. Recent advances in peptide-mediated regulation of root development. CLE, RGF/GLV/CLEL, IDA, and CEP peptides are involved in several aspects of root development including lateral root formation (A), (B), (D), (E), protoxylem development (F), stem cell maintenance (G), and gravitropic response(C). Known pathways discussed in this review are shown near their approximate location in the root. Peptides are indicated in green text, receptors in blue. Upstream processes are yellow and downstream processes are red. Developmental output is indicated by black text. http://www.frontiersin.org/
Auxin and Cytokinins interactions:
In plants each of the phytohormones, though have independent effects, often they affect the other or they may have synergistic effect. For example Auxin prepares the cell but cytokinins execute cell division. When auxin induces new root formation at a particular concentration, cytokinins inhibit auxin induced new root formation.
Cross talk between stress and hormones through signalling.
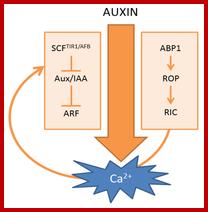
Scheme of auxin-induced Ca2+ signals. (Left) Canonical SCFTIR1/AFB-mediated auxin signaling; (Right) ABP1-mediated auxin signaling. The curved arrow represents a hypothetical model in which Ca2+ acts as a connecting signal between ABP1 and SCFTIR1/AFB signaling cascades. http://www.mdpi.com/
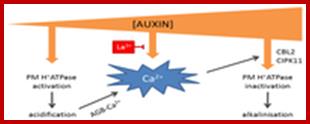
Hypothetical model of auxin concentration-dependent control over apoplastic pH. At low concentrations, auxin activates plasma membrane (PM) H+ ATPases, thereby lowering apoplastic pH and increasing apoplastic Ca2+ concentrations via arabinogalactan glycoproteins (AGBs). At high auxin concentrations, auxin induces a Ca2+ signal that inactivates H+ATPases. The auxin-induced Ca2+ signal can be inhibited by La3+.http://www.mdpi.com/
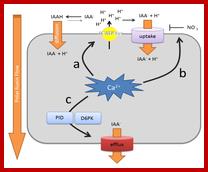
Summary of the effects of Ca2+ on polar auxin transport rates. (a) Cellular Ca2+ signaling impacts on auxin uptake mechanisms via effects on the abundance and activity of the plasma membrane H+ ATPase. The amount of protons in the apoplast determine the auxin uptake rate via diffusion of protonated indole-3-acetic acid (IAAH), as well as H+/IAA- (indole-3-acetic acid) symport; (b) Ca2+ can change the affinity of NRT1.1 for nitrate and auxin uptake; (c) Ca2+ controls the activity of the auxin efflux machinery by modulating the kinase activity of PINOID (PID) and, possibly, also D6PKs. PID also impacts on PIN-formed (PIN) polarity (not depicted). Uptake refers to active uptake mechanisms. Efflux refers to active auxin efflux mechanisms. http://www.mdpi.com/