Plant Development-Introduction:
Concept of Growth and Differentiation:
In Plants, besides vegetative propagation, production of a zygote to produce a zygote is two events separated in space and time. The zygote in an embryo sac divides and redivides to produce a mass of cells. Concomitant with the cell divisions polarity of future shoot apex and root apex is also determined and established which is accompanied with physiological and structural changes. After a period of active growth, the embryo suspends its active state and becomes dormant. At this stage, the embryo which is enclosed in a seed coat is shed from the mother plant. Then after a period of rest, which may be short or long, if conditions are favorable, the seed germinates and develops into a full fledged plant. Such plants inturn, after going through a period of vegetative development produce reproductive structures in which once again a zygote develops due to the act of fertilization. And the cycle is repeated. Between these two stages the time required for the development varies from as short as 24 hours, to several years. This is however determined by the genetic potentiality of the given plant, but environmental factors have a strong and stimulating influence on the plant genome for its expression and manifestation of the system.

Apical meristem derived cells and tissues; www.vialattea.net
A careful analysis of various events in the development of higher plants reveals that there are two important processes that go hand in hand. One is growth increase in size or volume and the other is differentiation; together it is called development. Growth is generally defined as an irreversible increase in size/volume accompanied with an increase in the protoplasmic mass. Differentiation, on the other hand, is a process in which new structures with specific functions generate from the preexisting cellular components. For that matter, these two events are not mutually exclusive.
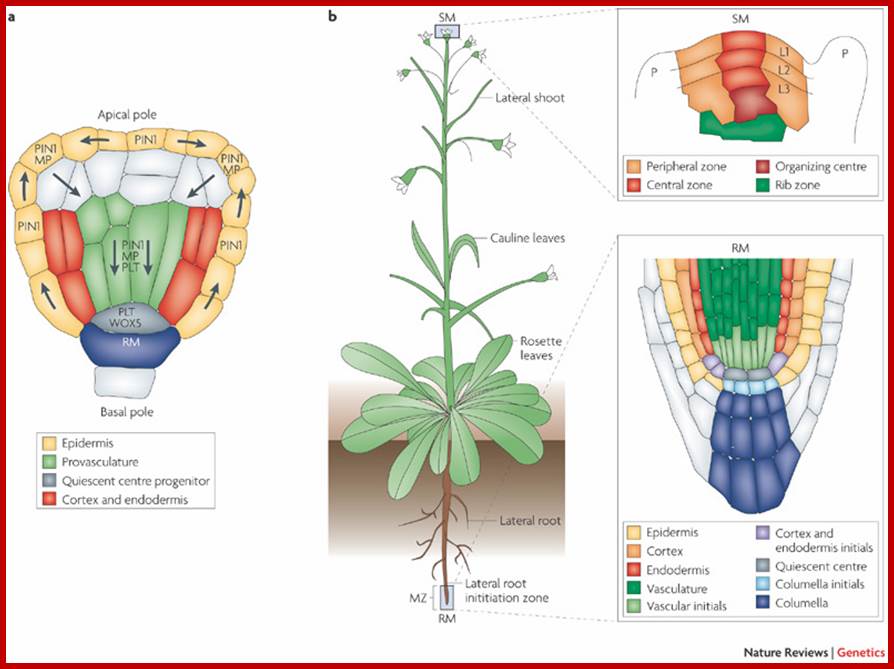
Plant embryo in globular stage: Direction of Auxin flow requires Monopetros (MP), MP promoted expression of Auxin efflux protein PIN-FORMED1in provascular cells. The direction of transport is shown; this leads to accumulation of auxin at the basal pole where the root meristem is established. Plethora (PLT) and WUSCGEL-related homeobox 5 (WOXS) are MP dependent transcription factors.
Right-The location and structure of root meristems illustrated in the right side expanded boxes. L1,L2 and L3 meristem layers; MZ; Meristamatic zone, P-primordium at initiation site.http://www.nature.com/;
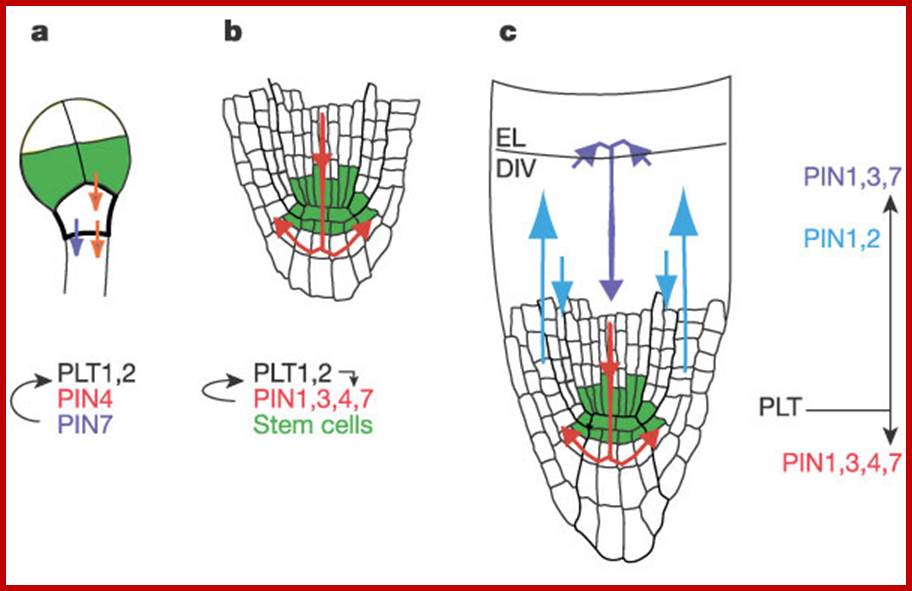
The PIN auxin efflux facilitator network control growth and patterning in Arabidopsis;Ikram Billou et al; a, PIN-mediated root primordium specification by restriction of PLT transcripts in octant/16-cell embryo stage. b, At later stages of embryogenesis, PIN action further restricts PLT transcripts to define the stem cell region and PLT genes start controlling root-specific PIN gene expression. c, In post-embryonic roots, PIN-mediated auxin transport stabilizes the stem cell region and regulates cell division (DIV) in the meristem zone and cell expansion in the elongation zone (EL). PLT genes control several members of the PIN gene family to generate primordium-specific auxin distribution. www.narure ,com
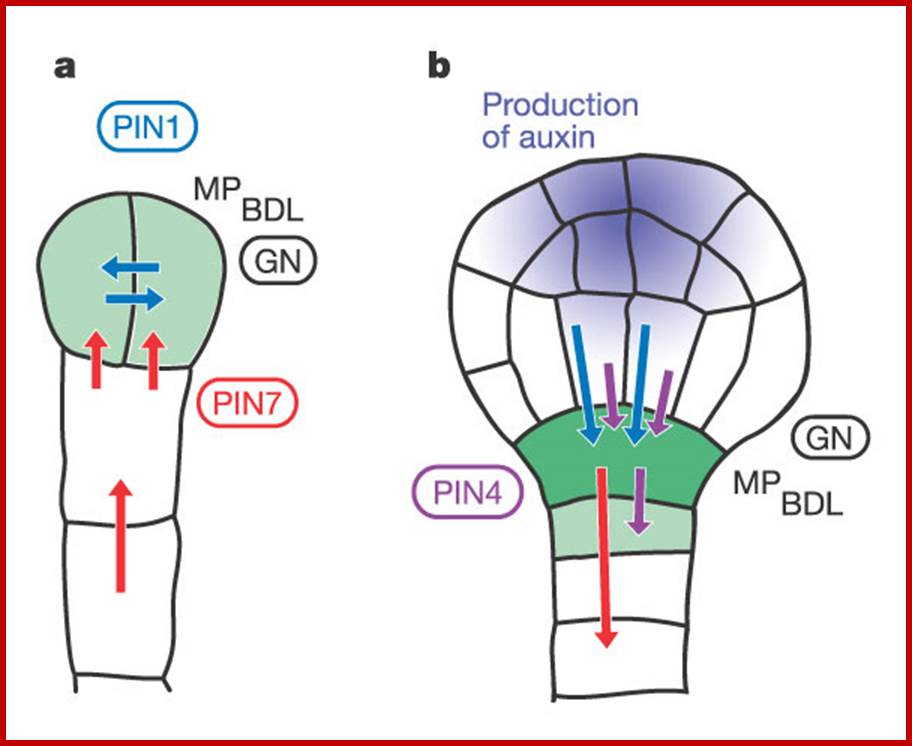
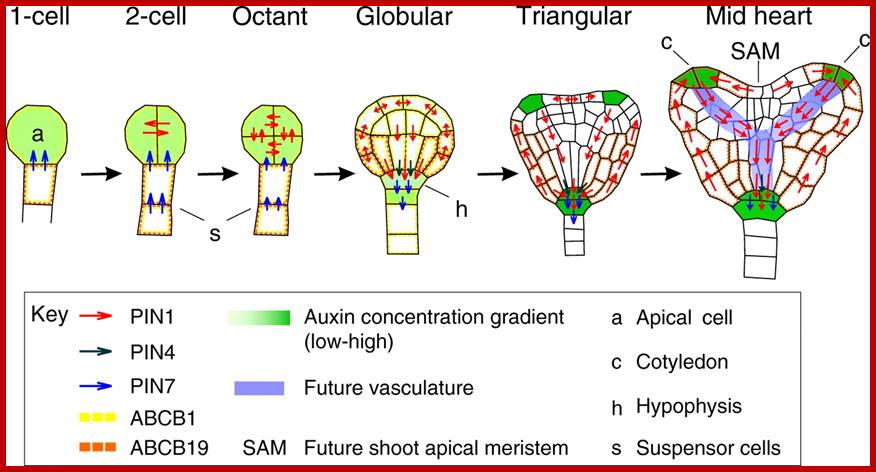
A model for a role of Auxin in embryo patterning; Sites of auxin response and accumulation are shown in green. Arrows indicate routes of auxin efflux mediated by PIN1 (blue), PIN4 (purple) or PIN7 (red). Also depicted are proteins involved in embryo patterning and related to auxin transport (GN, encircled) or response (BDL, MP). a, Two-cell-stage embryo. Auxin accumulates in the proembryo through PIN7-dependent transport and triggers apical pole specification. b, Young globular embryo. Free auxin starts to be produced in the apical part (purple) and auxin transport routes reverse. Auxin accumulates in a PIN1- and PIN4-dependent manner in the hypophysis, triggering root pole specification.; this then precedes the development of plant. Friml et al; http://www.nature.com/
Auxin transport route. www.fevelopment.biologist.org
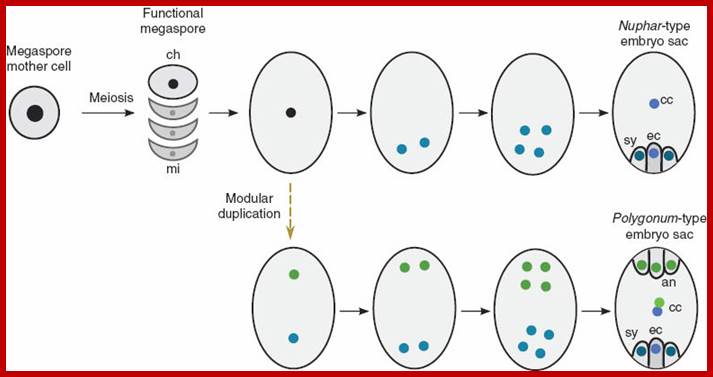
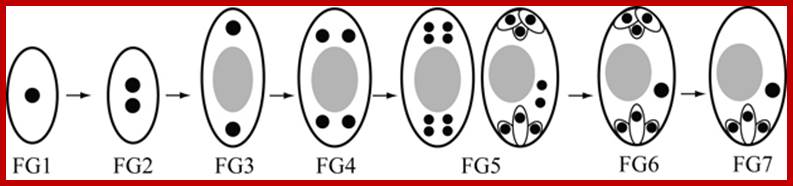
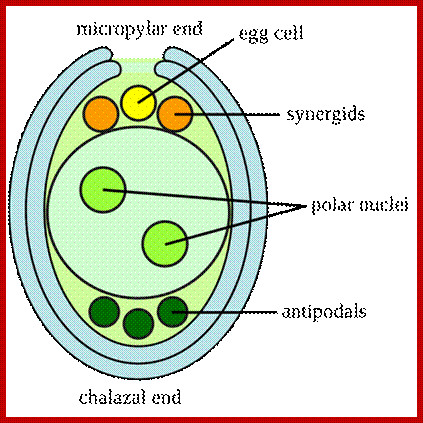
Early embryo sac differentiation; https://faculty.unlv.edu/schulte/
Development of embryo sac with primary endosperm nucleus in the center and egg on the opposite side at the pollen tube entry site with synergids on either side and at the opposite pole three nuclei form antipodal cells; http://www.degruyter.com/
http://people.emich.edu/

http://people.emich.edu/ and https://www.quora.com/
The female gametophyte consists of two synergid cells, two cells in the center; one egg cell and the other called central cell and three antipodal cells at the opposite end of embryo sac. No specific functions have been attributed to antipodal cells, but some have found it undergoes programmed cell death (apoptosis) during embryo sac maturation before fertilization. Some have observed the living antipodal cells beyond fertilization. Some have found them to acquired glandular properties. Also they contain mitochondria, ribosomes, plastids in antipodal as well as synergid and egg cells. The central call contains two nuclei they fuse before fertilization; The central cell contains two nuclei and cytoplasmic organelles as one male cell fuses with egg cell and the other cell fuses with the central cell to form triploid cell, which divides and redivides after fertilization into endosperm cells.
We report that an egg-cell-specific gene, ZmEAL1, is activated at the micropyle pole of the eight-nucleate syncytium. ZmEAL1 translation is restricted to the egg cell, resulting in the generation of peptide-containing vesicles directed toward its chalazal pole. RNAi knockdown studies show that ZmEAL1 is required for robust expression of the proliferation-regulatory gene IG1 at the chalazal pole of the embryo sac in antipodal cells. We further show that ZmEAL1 is required to prevent antipodal cells from adopting central cell fate. We further show that ZmEAL1 is required to prevent antipodal cells from adopting central cell fate. These findings show how egg cells orchestrate differentiation of the embryo sac. Nadia Graciele Krohn; http://www.cell.com/
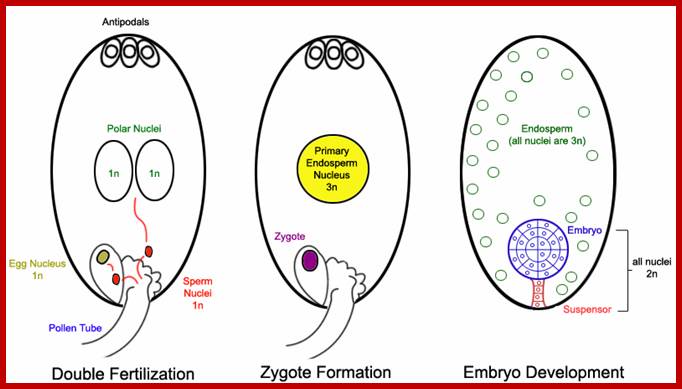
Pollen tube delivers two nuclei from male; of which one fuses with central nucleus another fuses with the nucleus located adjacent to two nuclei; the fertilized nuclei start dividing into an embryo and the secondary nucleus divides and redivides to generate endosperm.; http://www.biosci.ohio-state.edu/
Synergid cells; they are found on either sides of the egg cell which help or guide pollen tube in the female gametophyte; Greek synergos- working together; Eduard Strasburger noted that these cells somehow assist fertilization of the egg. They have thickened cell walls produce filiform apparatus at the micripylar end; which increase surface area of cytoplasm in synergid cells. R2R3 Myb9 protein MYB98, it binds to DNA involved in expression of at least 16 synergid genes, thus it is concluded the mYB98 is key regulator of transcriptional events in synergid cells which help in guiding male game cell toward central egg cell. During the entry of the pollen tube enter synergid cells and synergid cells collapse. Once the pollen tube enters its growth is arrested and, the tip breaks and two sperm cell are released; the synergid cells helps or guide the sperm cells toward the central egg cell.
The Levels of Male Gametic Mitochondrial DNA Are Highly Regulated in Angiosperms with Regard to Mitochondrial Inheritance;
Dan-Yang Wang,a,1 Quan Zhang,a,1 Yang Liu,a,1 Zhi-Fu Lin,b Shao-Xiang Zhang,c Meng-Xiang Sun,d and Sodmergena
The mechanisms that regulate mitochondrial inheritance are not yet clear, even though it is 100 years since the first description of non-Mendelian genetics. Here, we quantified the copy numbers of mitochondrial DNA (mtDNA) in the gametic cells of angiosperm species. We demonstrate that each egg cell from Arabidopsis thaliana, Antirrhinum majus, and Nicotiana tabacum possesses 59.0, 42.7, and 73.0 copies of mtDNA on average, respectively. These values are equivalent to those in Arabidopsis mesophyll cells, at 61.7 copies per cell. On the other hand, sperm or generative cells from Arabidopsis, A. majus, and N. tabacum possess minor amounts of mtDNA, at 0.083, 0.47, and 1 copy on average, respectively. We further reveal a 50-fold degradation of mtDNA during pollen development in A. majus. In contrast, markedly high levels of mtDNA are found in the male gametic cells of Cucumis melo and Pelargonium zonale (1296.3 and 256.7 copies, respectively). Our results provide direct evidence for mitochondrial genomic insufficiency in the eggs and somatic cells and indicate that a male gamete of an angiosperm may possess mtDNA at concentrations as high as 21-fold (C. melo) or as low as 0.1% (Arabidopsis) of the levels in somatic cells. These observations reveal the existence of a strong regulatory system for the male gametic mtDNA levels in angiosperms with regard to mitochondrial inheritance. The reduction of mtDNA labeling in the pollen cells was once more detected and thus verified that microscopic examination of mtDNA quantity correlates with the results of molecular analysis. It is clear, therefore, that the level of pollen mtDNA is down regulated during pollen development in Arabidopsis, In plants, in most of the cases plastids are inherited through females than male components that is pollen, however some percentage of plants show biparental inheritance of plastids. Uniparental inheritance of plastids offers an advantage in creation and cultivation of genetically modified plants (prof.Shekar Ali). The first documentation of male sterility was by Joseph Gottlieb Kölreuter, who observed anther abortion within species and specific hybrids. Cytoplasmic male sterility has now been identified in over 150 plant species. Cytoplasmic male sterility is being exploited by plant breeders (Wikipedia).
Note; on the contrary in animal systems sperm does not carry any mitochondria, only female egg cells carry mitochondria; so mitochondria are inherited along female lines (uniparental)..
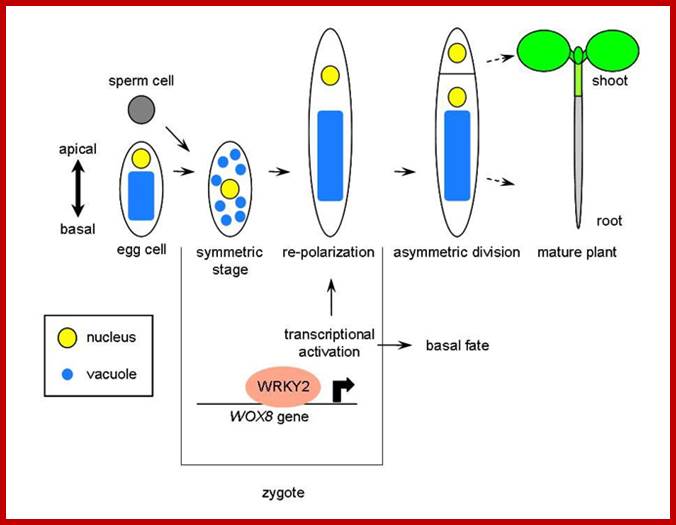
Transcriptional activation of Arabidopsis axis patterning genes WOX8/9 links zygote polarity to embryo development: Ueda M, Zhang Z, Laux T.; http://old-site.bioss-freiburg.de/
Cell-to-cell communication is integral to the evolution of multicellularity. In plant development, peptide signals relay information coordinating cell proliferation and differentiation. These peptides are often encoded by gene families and bind to corresponding families of receptors. The precise spatiotemporal expression of signals and their cognate receptors underlies developmental patterning and expressional and biochemical changes over evolutionary time have likely contributed to the refinement and complexity of developmental programs. Here, we discuss two major plant peptide families which have central roles in plant development: the CLAVATA3/ENDOSPERM SURROUNDING REGION (CLE) peptide family and the EPIDERMAL PATTERNING FACTOR (EPF) family. We discuss how specialization has enabled the CLE peptides to modulate stem cell differentiation in various tissue types, how differing activities of EPF peptides precisely regulate the stomatal developmental program, and we examine the contributions of these peptide families to plant development from an evolutionary perspective.
Increase in the number of cells accompanied with the increase in volume has an apparent effect on the increase in the size of the plant body. The same can be determined by either counting the number of cells or measuring the volume or both. But differentiation involves the molecular changes in undifferentiated cells, leading to the formation of new structures, like tissues, leaves, buds, branches, flowers and fruits. So the development can be considered as the summation of all physiological activities.

Organization of Apical meristem-1.central zone, 2.Peripheral zone, .Medullary meristem, 4. Medullary tissue.;www.eplantscience.com
www.geneseo.edu ; http://pgjennielove.files.wordpress.com/
Measurement of growth:
Normally growth is measured in terms of increase in the number of cells, increase in height or weight with time. These can be determined by known physical methods. Even the number of leaves, branches, roots, flowers and fruits produced can be measured by simple methods. The developments of later said structures are the products differentiation which can be further subjected to quantitative or qualitative studies.
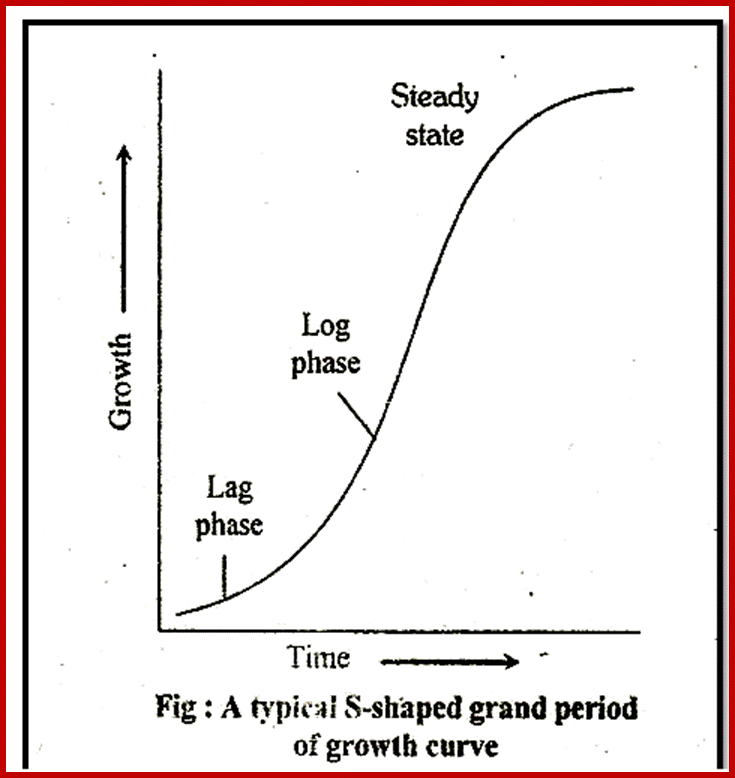
The growth of a seedling, in terms of increase in length or weight, can be plotted as the function of time which may be in hours or days. The graphical expression, which results in a curve called growth curve. Different plants, for that matter different organ of the same plant, exhibit different kinds of growth curves. For example, a tree plant increases in height and weight with time. Similarly, the number of leaves, flowers and fruits produced show different pattern, which is quite distinct from that of growth pattern of a single leaf, flower or fruit. The plants exhibit indefinite growth and determinate growth pattern. But in both one can observe the growth pattern which passes through three different phases one leading to the other. They are lag phase, log phase and steady or constant phase.
Growth curve –in general; http://www.askiitians.com/
http://www.vwmin.org/
It is possible to utilize the above said growth curves in determining overall growth rate or the growth rate of individual phases. This can be done by taking a tangent angle degree derived from two points on the curve, which provides the information about the increase in length or weight in a given period of time. By integrating them together, one can obtain the growth rate. The same value i.e. growth rate (increase in length or wt.xTime) can be plotted against a larger time scale. This plot gives a general pattern of growth rate at different phases of growth of the whole plant. The finer analysis of these growth patterns can be employed to determine the efficiency of plants in terms of its increased or deceased dry matter, photosynthetic efficiency, flowering, fruit production, etc.
In fact, using the above methods, it is also possible to determine relative growth rate, not assimilation rate and the ratio of leaf area to dry weight or leaf area ratio. The above methods provide an invaluable data in determining the genetic potentiality of plants in response to different environmental conditions. The efficiency of the plants in terms of input and output can also be determined.
Growth Rate and Phases of Growth:
Fate of SAM products; novszerv.elte.hu
Different plants exhibit different growth rate; this is the law of nature. Generally, most of the plants initially show a period of slow growth. This is called lag phase. At this stage most of 5he available reserve food material is metabolized to generate energy rich compounds and various organic compounds necessary for building the structural components of cells. In this period, most of the reserve food material is drawn into metabolic pool.
Further development depends upon the ability of the plants to synthesize its own food material, which is achieved by the development of leaves. Once sufficient number of leaves and roots are produced, the plant becomes self sufficient and then the plant grows at a faster pace. In fact, the increment of growth doubles with time. This phase of growth is called log period or exponential period of growth.
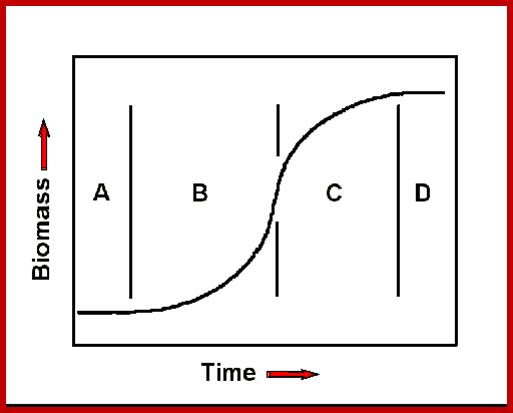
Phases of growth- Phases A, B, C and D, lag. Exponential transitional and equilibrium phases respectively; http://www.mobot.org/
The term exponential is derived from the fact, the increase in the number of cells or increase in height or weight, when measured at equal periods of time always doubles, etc., 1, 2, 4, and 8, 16, 32, 64 and so on. Such a relationship between the number of cells produced in a given time is expressed in the form of an equation i.e. N=4t, where t is the time interval and N is the number of cells or weight or length. If the growth pattern obeys the said equation, then it is called exponential growth. If fact, it is at this period of time, the plant reaches the maximum differentiation and development, in terms of production of leaves, buds, branches, etc., and the plant gains maximum height and weight.
The magnitude of exponential growth in this period however, varies from plant to plant. For example, Calamus gigantica (bamboo) grows at the rate of 2 ft./day, Asparagus at 1 ft/day, inflorescence axis in Agave 6 inches/day, Basella bacella 2 inches a day. On the contrary Cycas, a gymnosperm, grows at the rate of 1.2 to 2 inches/year. The above examples clearly indicate that the ability of growth of each plant is ultimately determined by its genetic potentiality.
Once plants reach their maximum growth point, they start producing flowers. This of course varies from species to species. With this, the growth rate of plants falls sharply, but the other non flowering branches continue to grow but slowly. Thus a steady rate of growth is maintained. Such a phase is called steady phase. But I may annuals, with flowering and fruiting, the growth comes to stand still and ultimately the plant dies. Nevertheless, the perennial plants (herbs, shrubs or trees) exhibit further seasonal vegetative growth and seasonal flowering/fruiting periods, where the growth curve is different from that of annuals. Such a behavioral pattern in exhibited by evergreen plants as well ad deciduous plants.
While the above observations are true for the entire plant, the growth rate of leaves, flowers and fruits exhibit a different pattern. They too have a lag phase, a log phase and constant phase where the growth of the said organs is virtually stopped. All structures or organs which have determinate growth show this pattern. Furthermore, the magnitude of growth of different organs varies from species to species.
Mechanism of Growth and Differentiation:
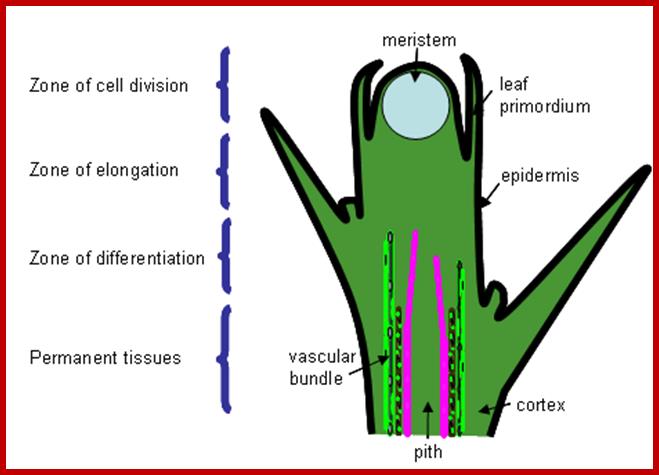
It is very important to understand the regions of growth that are involved in the development of the plant body. This can be taken as the basis for understanding the mechanism of growth and differentiation. The apices of shoots and roots provide valuable information about the structures involved in growth and differentiation of cells during development of plant structures. Refer to the topic cell differentiation as the function of gene expression to understand the molecular basis of differentiation.
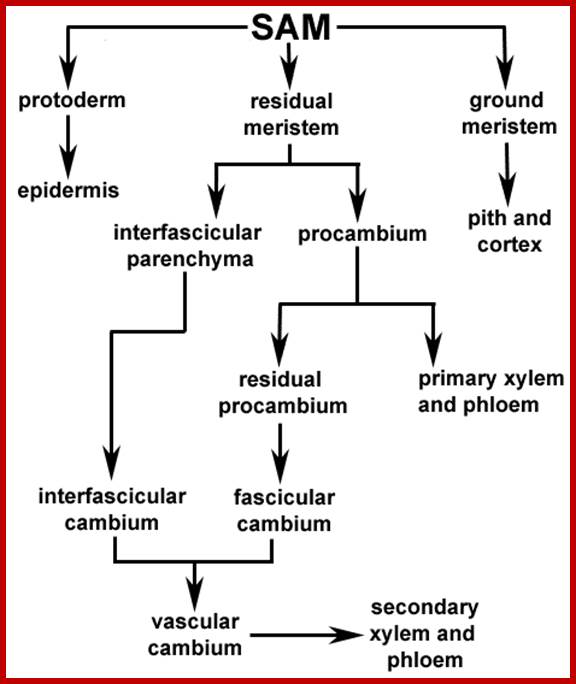
Both root apex and shoot apexes are made up of three important regions i.e. the region of meristems, the region of elongation and the region of differentiation. The meristematic region is restricted to the apexes, where the cell will be in an active state of cell division. In the region of elongation, cells are in the process of physical elongation, thus less region exhibits an apparent growth. Just behind this region, the elongated cells or the cells that are still in the process of elongation show marked differentiation in their cellular structures and functions. In this region, one can see different types of cells and tissues like pith, xylem, phloem, endodermis, cortex etc. The analysis of each of these structures and their functions will give a comprehensive picture of how cells grow, differentiate and produce specific organs.
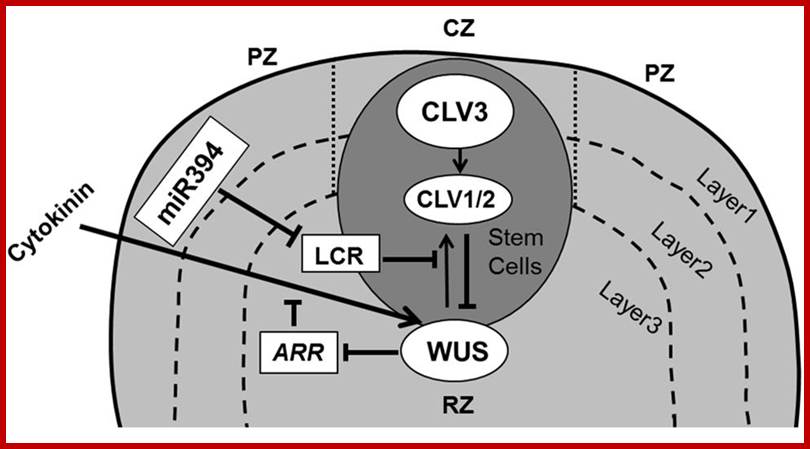
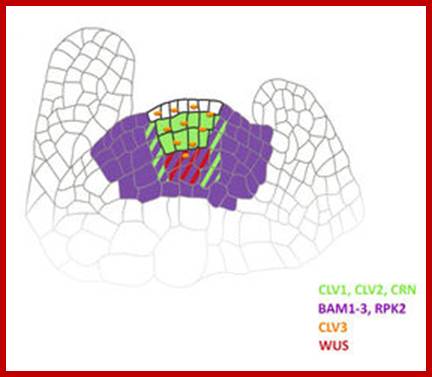
Maintenance of stem cells in shoot apical meristem; The SAM is organized in three functional zones [central zone (CZ), peripheral zone (PZ), and rib zone (RZ)] and three layers where the antagonistic relation between WUS (WUSCHEL) and CLV (CLAVATA) is essential to preserve cells in the meristem. WUS activates CLV3, which further binds with CLV1/2 and in turn inhibits expression of WUS. Cytokinin positively controls WUS expression where ARRs are negative regulators of cytokinin and are inhibited by WUS. The L1 specific miR394 negatively affects the LCR protein, which interferes in WUS/CLV based stem cell maintenance (pointed and T shaped arrows indicate positive (WUSCHEL( and negative (CLAVATA) regulation, respectively).WUSCHEL- positive http://journal.frontiersin.org/
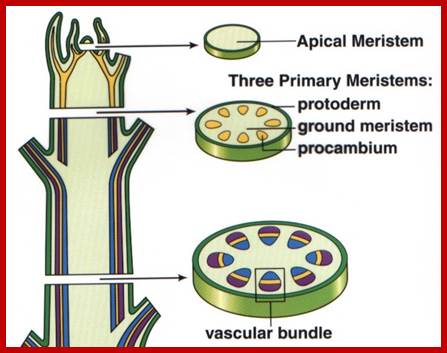
Meristems: Karl Willhelm von Nageli used the term Meristem for the first time in 1858. The higher plants, in their embryonic state itself, acquire polarity for their future development. Undifferentiated Meristem cells are similar to animal Stem Cells for they can be induced to divide and redivide and differentiate into specific organs. The embryo possesses plumule at one end and radicle at the other end of the plant axis. The plumule develops into shoot system and radicle into root system. But shoot apex and root apexes contain a single or a group of meristematic cells. Each cell in this region is endowed with a potentiality of active cell division and this potentiality is retained throughout the lifetime of the plant. Such meristematic cells are rich in cytoplasm, compactly arranged with a number of intercellular protoplasmic strands as interconnecting structures. Vacuoles in these cells are nearly absent. Nucleus is active. Cells exhibit maximum respiratory activity to generate energy rich compounds. Other metabolic pathways are very active in generating various compounds to build up various cellular components and structures. The nuclear DNA undergoes periodic replication and various sets of genes are expressed in producing required TRNAs, RRNAs and RNAs. Once these RNAs reach the cytoplasm, they are utilized in synthesizing a host of proteins of which some are used in the organization of different cellular structures like membranes of ER, plasma lemma, mitochondria, plastids, nucleus, lysosomes, golgi bodies, cytoskeletal structures, microtrabaculae, chromatin, ribosomes, etc. and other proteins act as enzymes and regulatory factors for various metabolic processes. These activities in meristatic cells are maintained as well as sustained till the death of the plant. Except under dormant conditions where all the cellular activities are temporarily suspended, they are fully active throughout the life of the plant.
Shoot stem of Arabidopsis; http://www.nature.com/www.devgen.hhu.de
http://mplant.oxfordjournals.org/
Molecular Mechanisms of Auxin and Cytokinin Interaction in the Regulation of Plant Meristem Development;
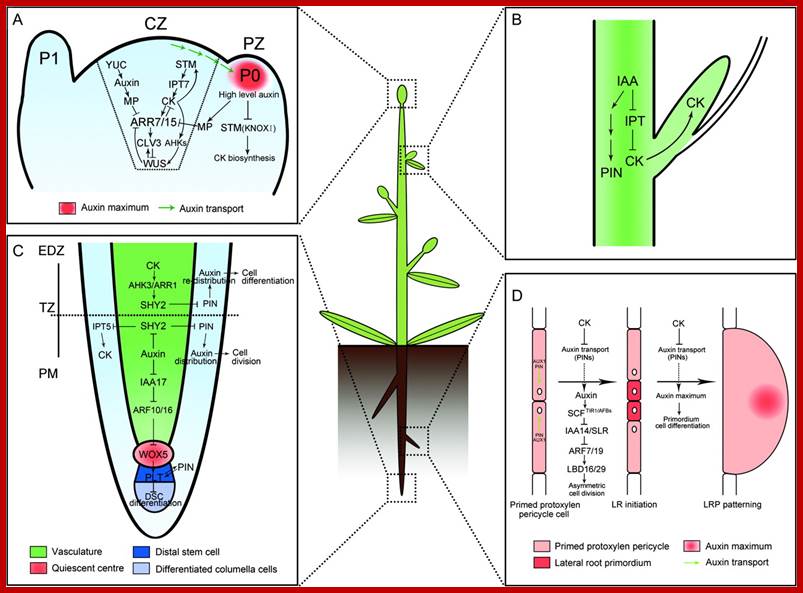
(A) In CZ of shoot meristem, ARR7 and ARR15 act as integrative factors in auxin and cytokinin signaling pathways. Auxin represses the expression of ARR7 and ARR15 while cytokinin promotes their expression through a STM-dependent pathway. Both of them regulate the expression of WUS in a negative feedback loop, critical for stem-cell formation. During the formation of lateral organ primordia, a high level of auxin transported from CZ blocks the biosynthesis of cytokinin by suppressing KNOXI function in PZ. CZ, central zone; PZ, peripheral zone; P0/P1, organ primordia.
(B) Auxin is transported from the shoot apex to repress cytokinin biosynthesis, leading to the inhibition of axillary bud growth.
(C) In the root meristem, auxin promotes the expression of PINs through the degradation of SHY2 proteins, resulting in the maintenance of an auxin gradients and cell division. In contrast, cytokinin impedes the expression of PINs by stimulating the expression of SHY2, leading to auxin redistribution and cell differentiation. Auxin also plays an important role in the differentiation of root DSC by mediating the expression of WOX5 and PLT. PM, proximal meristem; EDZ, elongation differentiation zone; TZ, transition zone; DSC, distal stem cell.
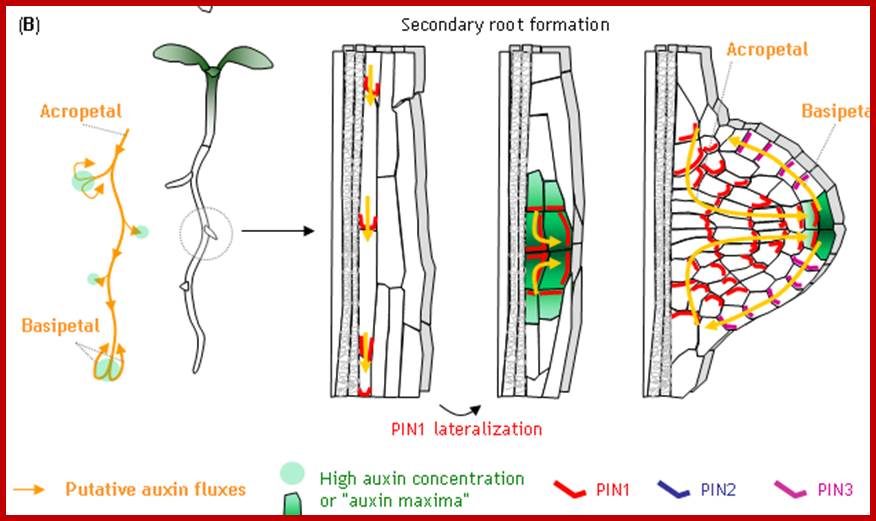
(D) In certain xylem pole pericycle cells, the transport and perception of auxin trigger an asymmetric cell division critical for the LR initiation and LRP patterning. By contrast, cytokinin negatively regulates the LR initiation and LRP patterning by inhibiting the expression of PINs and the auxin distribution gradients. LR, lateral roots; LRP, lateral root primordia.
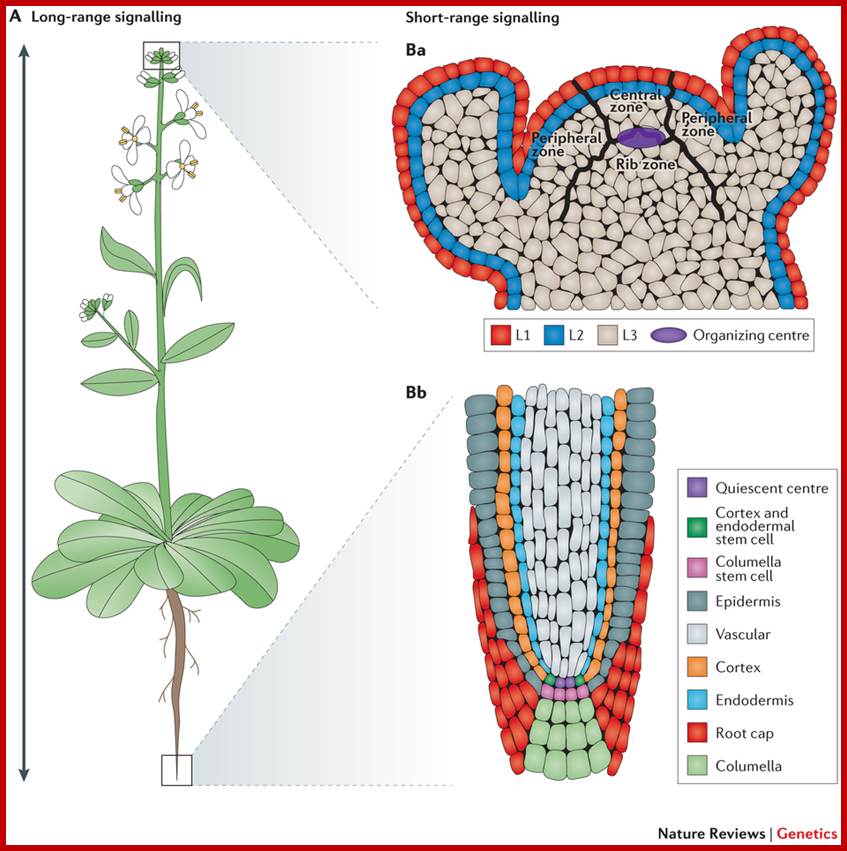
Arabidopsis thaliana is a dicotyledonous plant that is used as a model organism for developmental studies. Two classes of signalling are used in A. thaliana: long-range signalling encompassing travel over a considerable distance (for example, shoot to root); and short-range signalling involving local intercellular movement. A. thaliana organs originate from two meristem populations, one in the shoot and the other in the root. Both meristems contain niche cells and stem cells or initial cells. However, each meristem has a distinct organization and cell types that arise from it. The shoot apical meristem (SAM) contains three layers (L1, L2 and L3) and three developmental zones (peripheral zone, central zone and rib zone). The niche cells or organizing center, of the SAM are specified at the junction of the three developmental zones and function to maintain stem cells in the shoot. The root apical meristem (RAM) (part Bb) is radially symmetric and consists of central niche cells (the quiescent centre) surrounded by stem cells. In the root, each stem cell population gives rise to one or two cell types. Nature Reviews Genetics
Shoot stem cells of ARABIDOPSIS are controlled by the CLAVATA pathway; the shoot meristem generates the above-ground plant organs. In the model plant Arabidopsis thaliana, stem cells reside at the meristem tip. Their fate is controlled by cells in a deeper region, the organizing center. Stem cells secrete the short peptide CLAVATA3 (CLV3) which is perceived by the receptor kinase CLAVATA1, and a heteromeric complex formed by the receptor protein CLAVATA2 (CLV2) together with the kinase CORYNE (CRN). This signal is transmitted from the cell-surface receptors to the nucleus to alter the expression levels of transcription factors, such as WUSCHEL (WUS). Because WUS promotes stem cell fate at the meristem tip, a feedback circuitry is established that regulates stem cell homeostasis. Other receptors contributing to these signalling pathways are the BAM receptors, and RPK2.
In recent years, we have studied how these receptor proteins interact at the plasma membrane and signal to the nucleus, and how functional receptor complexes assemble in the ER. We are using a range of different methods to unravel these receptor interactions in vivo. Very important tools are fluorescence imaging technologies and confocal microscopy; Heinrich Heine; http://www.devgen.hhu.de/Heinrich;
Selecting organ founder cells:

Organs such as leaves or flower organs are initiated at the flanks of shoot or floral meristems in a regular pattern. A key factor that positions organ founder cells is the phytohormone auxin, which is dynamically redistributed during plant growth via the activities of local auxin influx and efflux carriers. Organs generally initiate where auxin accumulates; at these sites, the expression of meristem specific genes is repressed. Several genes belonging to the LATERAL ORGAN BOUNDARY DOMAIN (LBD) family of transcription factors are required to maintain the boundary between organs and the remainder of the meristem, and thereby restrict cells with stem cell fate and differentiating cells to separate, but adjacent domains.
The gene JAGGED LATERAL ORGANS (JLO) belongs to the LBD family and controls both meristem specific gene expression patterns, but also auxin transport and signalling in root and shoot development. We are studying JLO function and interactions to learn how differentiation of meristem cells is controlled, and how organ initiation patterns are determined.
Cell Division and Plane of orientation:
Once the cellular components are made and the protoplasmic mass reaches a particular proportion in terms of mass, the cells initiate mitotic divisions. This results in the production of daughter cells. Some of the daughter cells which are away from the mother meristem cell act as derivatives. The most crucial part of mitotic divisions is the plane of division. This is however determined by the plane of orientation of mitotic apparatus in the dividing cells. As shown in the fig upper cells undergo another period of preparation for another cycle of cell division to produce another set of derivative cells and the process goes on and on thus the terminal cells fund in the apical dome continue to divide and redivides ceaselessly to generate new cell derivates. The regulatory factors that control cell divisions continue to be produced in mitotically active meristematic cells there by the said cells keep on their mitotic activity unhindered. On he contrary, the cell derivatives produced by the upper meristematic cells, because of their positional effect, nutritional and hormonal influences produce certain regulatory factors some of which block gene expression required for continuous mitotic activity and others stimulate the cells to undergo certain transformation leading to differentiation.
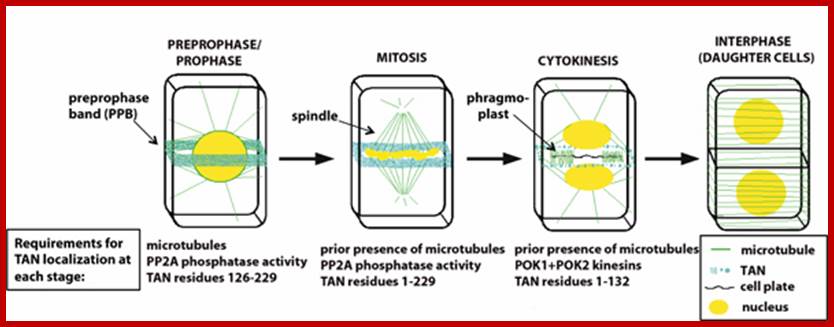
Schematic of cytokinesis in a somatic plant cell; TANGLED protein (blue) co-localizes with the preprophase band (PPB), predicting the future division plane during prophase. Unlike the PPB, TAN remains at the cortical division site throughout mitosis and cytokinesis, where it functions to promote proper placement of the new cell wall. Maintenance of TAN at the division site requires different parts of TAN protein and different interacting factors at different stages of the cell cycle. http://biology.ucsd.edu/
Mutation studies in yeast cells clearly suggest that mitotic cycle is controlled by at least 36-38 mutational sites an each one of them are distinct and specific in their activity. Some of the genes, if activated, may block the continuous mitotic activity. On the other hand, if some specific regulatory genes undergo mutation, it induces uncontrolled mitotic activity which may lead to cancerous growth. However in higher plants to keep pace with the increase in the number of meristematic cells, the tunica layer of cells which cover the apical dome divide and redivides radially. It is interesting to note that the outer tunica layer of cells or epidermal cells are also meristematic but their plane of cell division is always radial. The intrinsic factors that are responsible for such a behavior of these cells are perplexing and fascinating. Nothing is known about this phenomenon.
Asymmetric Cell Division:
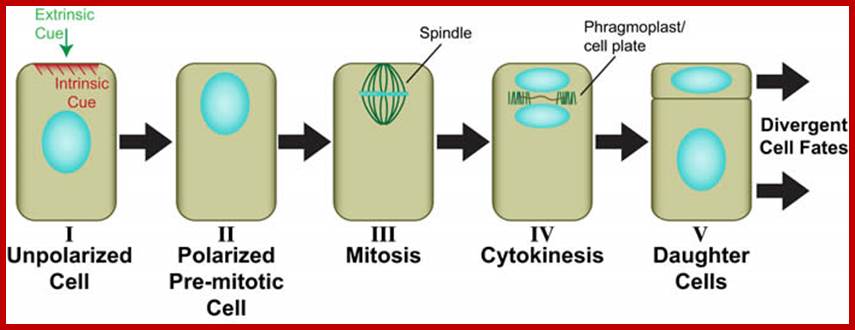
Generic view of plant asymmetric cell division (Facette and Smith, 2012). Perception of an intrinsic or extrinsic polarizing cue (I) leads to polarization of the mother cell including migration of the premitotic nucleus into the future division plane (II). The dividing nucleus is retained in the asymmetric division plane (III), where a new cell wall (cell plate) ultimately forms during cytokinesis through the action of the phragmoplast (IV). Daughter cells with different sizes and/or shapes are formed (V), which will adopt different developmental fates. http://labs.biology.ucsd.edu/
Asymmetric cell divisions are those that produce daughters of different sizes, shapes and/or developmental fates (see illustration below). In plants, as in other eukaryotes, asymmetric divisions are associated with pattern formation during embryogenesis, establishment of new cell lineages, formation of specialized cell types, and maintenance of stem cell populations. In all of these processes, developmental asymmetry is closely tied to division polarity, which is oriented by either intrinsic or extrinsic cues. Most work on asymmetric cell division in plants has focused on how the divergent fates of daughter cells are specified. In contrast, we are interested primarily in how the physical asymmetry of the division is achieved. For example, what are the polarizing cues? How are they perceived? How is this perception translated into premitotic polarization of the mother cell? How is the division plane oriented in relation to mother cell polarity?
Transitional Zone:
The basal cells though derived from the terminal meristematic cell(s) exhibit different pattern of growth. This is however, determined by the underlying mature or partially matures cells which supply nutrients in the form of mineral elements, organic compounds and plant hormones. The gradient and magnitude of various chemical components supplied from the lower cells have profound influence on the cells derived from meristematic cells. The positional effect of transitional zone cells, compounded by the hormonal and nutrient gradients the cells found in the transitional may undergo one or more cell divisions or they may directly undergo transformation leading to differentiation.
Onset of cell elongation and cell differentiation:
The cell derivatives thus produce, utilizing nutritional ingredients and phytohormones, undergo dramatic transformations. Not all the cells behave in the same way. Some elongate considerably and undergo transformation to produce different cell types, such as xylem elements, phloem elements, and endodermal cells and so on. Others without much elongation develop into cortical parenchyma, pith parenchyma and pericyclic cells. In stem apex, just below the meristematic zone cells, just below the tunica layer, some of the cells organize into leaf primordia, which actually cover the entire meristematic dome.
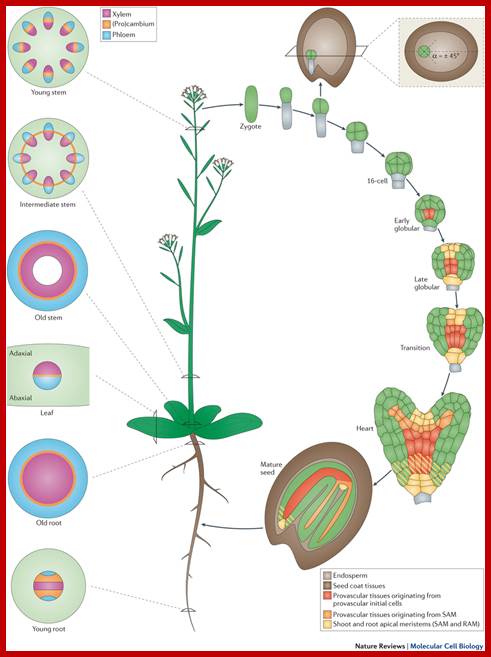
Plant vascular development: from early specification to differentiation;
Vascular tissues in plants are crucial to provide physical support and to transport water, sugars and hormones and other small signalling molecules throughout the plant. Recent genetic and molecular studies have identified interconnections among some of the major signalling networks that regulate plant vascular development. Using Arabidopsis thaliana as a model system, these studies enable the description of vascular development from the earliest tissue specification events during embryogenesis to the differentiation of phloem and xylem tissues. Moreover, we propose a model for how oriented cell divisions give rise to a three-dimensional vascular bundle within the root meristem. http://www.nature.com/
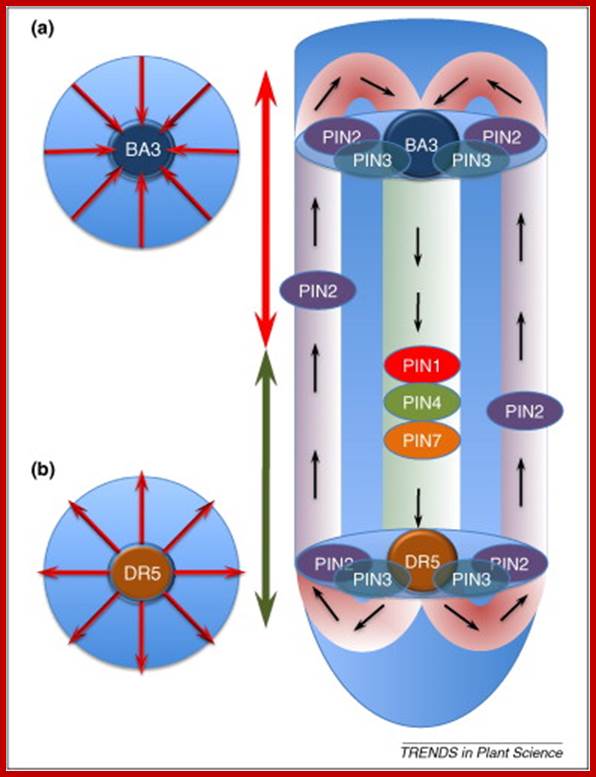
www.cell.com
Longitudinal zonation, as well as a simple and regular anatomy are the hallmarks of the root apex. Here the focus is on one particular root-apex zone, the transition zone, which is located between the apical meristem and basal elongation region. This zone has a unique role as the determiner of cell fate and root growth; this is accomplished by means of the complex system of a polar auxin transport circuit. The transition zone also integrates diverse inputs from endogenous (hormonal) and exogenous (sensorial) stimuli and translates them into signaling and motoric outputs as adaptive differential growth responses. These underlie the root-apex tropisms and other aspects of adaptive root behavior. w ww.cell.com
In roots, epidermal cells in the region differentiation elongate and develop into root hairs. However, among the vascular elements the first to be differentiated is phloem and then xylem elements develop. Though most of the cells are derived from the same group of meristems because of intrinsic factors, and programming the derivatives undergo transformation and develop into various cell types perform different functions.
Phloem as a Model for Understanding the process of differentiation:
Whether it is stem apex or root apex the first cell type to be differentiated are sieve tubes. The cells that are destined to become sieve elements are in line with the sieve cells of vascular bundles found below. The destined cell receives auxins from the cells found above and nutritional factors and sucrose from the lower vascular elements. Here sucrose appears to play a significant role in the formation of sieve cell. In response to hormones and nutritional factors, the said cell first undergoes an unequal cell division in the vertical plane. The smaller cell produced undergoes one or two divisions and develop into companion cells. But the larger cell undergoes remarkable transformation. First the cell elongates and expands. Concomitantly the nucleus disintegrates into small chromatin bits. It s at this juncture, the chromatin becomes active and produces substantial amount of mRNAs required for the formation of P-proteins and other factors. The mRNAs thus produced retain their translational activity for a period of 4-5 months. Once the p-proteins and its associated elements organize into longitudinally oriented tubular structures the tonoplast membrane disappears rendering the peripheral protoplasm and vacuolar materials, indistinguishable. The functional activity of such sieve tubes and companion cells are sustained for quite a long time.
Differentiation of xylem elements:
Similar to the development of sieve elements, some of the cells found by the side and just below the newly formed sieve tubes undergo differentiation and development into xylem structu5es. Experiments involving the induction of vascular elements in callus clearly show that the application of sucrose favor the development of sieve tubes and indole acetic acid on the other hand, stimulates the formation of xylem elements. So supply of IAA to these cells is crucial in the development of the xylem elements.
Responding to IAA and other nutritional factors, the cells that are destined to become xylem elements enlarge in length as well as in breadth. At the same time, the entire cellular machinery gears up to produce cell wall materials in massive amounts. Golgi bodies become very active, the vesicle loaded with cell wall components and enzymes are transported across the cell and the same are unloaded outside the plasma membrane. Synthesis and deposition of various components of secondary wall takes place on a large scale. Meanwhile endoplasmic reticulum starts associating with the plasma membrane at different sites. These regions become free from cell wall deposition and the same develop into pit canals. But in other regions, cell wall is deposited in a characteristic pattern in different cells of different xylem elements. During the development of xylem the protoplasm is completely used and finally it disappears; it is an apoptotic phenomenon, a programmed cell death. At the molecular level, it is clear that differential expression of genes is mainly responsible for the production of massive amounts of MRNAs required for the formation of enzymes needed for cell wall components. Even the deposition of cell wall material is regulated. Thus undifferentiated cells undergo differentiation into xylem structures to perform their specific functions.
Molecular Paradox in Morphogenesis:
During morphogenesis, the derivatives of meristems are subjected to nutritional and hormonal pressures. As a result cellular programs of these cells get modified due to the activity of regulatory factors. A set of genes which were active during early stages of mitotic cycle, at least, some of them get repressed, thus they switch off all the programmes for cycle events. At the same time, a new set of genes get activated which in turn exhibit cascade effect. In the sense, synthesis of a new group of mRNAs and their translation products activate another set of genes and such sequential gene inactivation and activation results in the transformation of undifferentiated cells into new cell types which by virtue of possessing a characteristic structure and functional potential perform specific functions; thus the division of labour sets into the system. There must be a master switch (s) in the form of regulatory factors that act at every stage of differentiation and growth. This can be similar to that of Homeobox or Hox gene clusters of animal system. Existence of such gene clusters have been discerned but not fully evaluated.
The regulation of gene expression may operate at the transcriptional level, translational level or at post translational level, but each of these events have their own feed back mechanisms. Besides nutritional factors and environmental factors phytohormones have a profound influence on gene regulation. Unlike animal systems plants have just a set of hormones like auxins, gibberellins, cytokinins, abscissins and ethylene. Among them the first three act as growth promoters and the others act as growth inhibitors. The site of synthesis, the time of synthesis, the concentration of each of these hormones at any given site and time, influence the molecular expression according to their specific effects. Though individual hormones elicit certain specific responses, their activity is greatly influenced by the presence of other hormones. One hormone in one tissue type may promote or inhibit the activity of the other hormone. Thus by modulating the levels of growth hormones, they induce different morphological structures. This is possibly achieved through a very complex gene regulation mechanism about which we know hardly anything.
Molecular aspects of IBA induced new root formation:
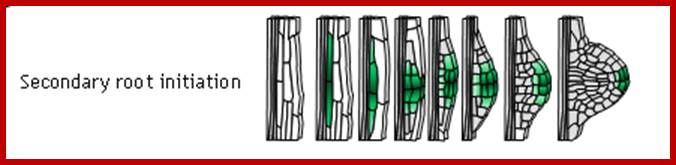
Adventitious root initiation in response to auxins like IBA has been used in understanding the molecular events that lead to redifferentiation in plants. In response to IBA, pericyclic cells found in the hypocotyl tissues of phaseolus vulgaris undergo transformation and reorganize into root primordial cells. The entire process takes place within 12-24 hours after hormone application.
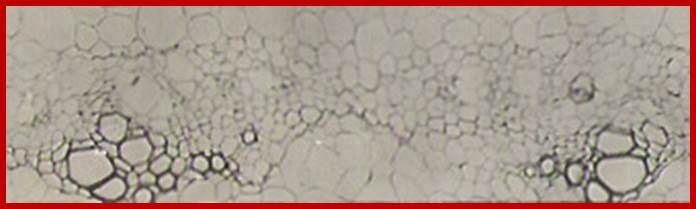
Pericyclic cells in between exarch tetrarch protoxylems, grkrRaj. PhD thesis

Pericyclic and inner layer of pericycle in between exarch protoxylems elements show large size with enlarged nuclei with large Nucleoli indicating cells programmed for cell division; GrkRaj. PhD Thesis.
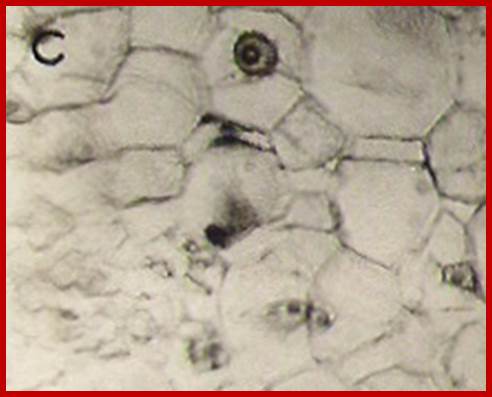
Large nucleoli suggest expression of rRNA in large quantity; GrkRaj-PhD thesis
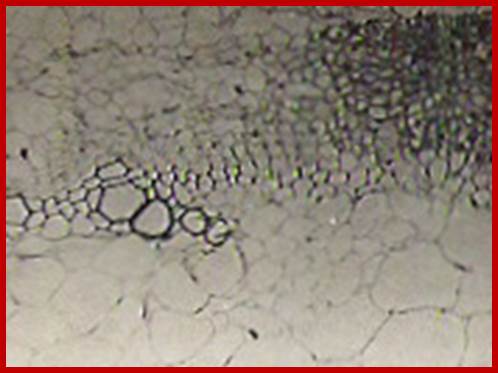
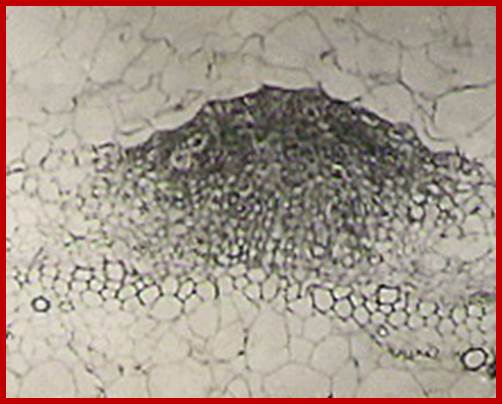
Cells in between protoxylem elements show large size and large nuclei indicating the cells have programmed for cell division in response to IBA treatment; around 36-48hrs after treatment one can observe root initials formed in between protoxylem elements; GrkRaj; PhD thesis;1979
Molecular events: Within 15-30 minutes of auxin treatment, even in the presence of actinomycin the rate of protein synthesis in the hypocotyls increases without any concomitant increase in mRNA synthesis, which suggests that early effect of IBA is on translational machinery. This view has been experimentally borne out by invitro translational studies. After another 60-90 minutes transcription and translational process further increase by 4-5 fold over control tissues. Among many changes observed in the pattern of proteins synthesized, the increased synthesis of 55-58 K Dalton proteins and another high molecular weight protein is very significant. Using SDS page, invitro translational system and immunoprecipitation methods the 55-58K protein has been identified as Tubulin.
New root formation in response to Auxin treatment; www.vwmin.org
Tubulin is known to be an important component of microtubule needed for mitotic apparatus and cytoskeleton structures. Furthermore tubulin polymerization into microtubules also increases in the presence of IBA and cytoplasmic factors. The above observations suggest that the IBA induced microtubules formation has some role in dedifferentiating the cells. In order to ascertain this view when the IBA pretreated hypocotyls are exposed to colchicine or cytochalasin B for a period of 36 hours, IBA mediated new root formation is completely inhibited, but after 36 hours of IBA pretreatment colchicine has no effect. The above results indicate that auxin mediated increase in tubulin synthesis, tubulin polymerization into microtubules, the organization and orientation of mitotic apparatus ultimately determines the plane of division. Besides another important observation is the transformation of pericycle cells into mitotically active cells. Thus IBA induces cell transformation and also determines the plane of division in parenchymatous tissue which ultimately determines the organization and development of root primordia.
Interestingly, the IBA mediated root initiation can be inhibited by higher concentration of cytokinins. Anatomical studies show that in the hypocotyls, treated with both auxin and cytokinins, the pericyclic cells show greater mitotic activity but root primordial organization is totally absent. But in the segments treated with cytokinin alone pericyclic cells do not show any changes.
In order to find out the interesting interaction between IBA and cytokinins and their effect on molecular events studies on the rate of protein synthesis and RNA synthesis show that the rate of protein synthesis increases as early as 15-30 minutes as cytokinin treated segments but the increase in RNA synthesis is delayed by 4-6 hours. Similarly in the hypocotyl which are exposed to both IBA and cytokinins, the rate of protein synthesis increases at 15-30 minutes, but the IBA mediated RNA synthesis is inhibited by cytokinin for a period of 4-6 hours. Later RNA synthesis and protein synthesis increase substantially in both the cases. Particularly in the stems treated with IBA+cytokinin, the increase is highest.
Analysis of in vivo protein pattern and invitro protein pattern reveals that though cytokinin increases the level of tubulin synthesis as in the case of IBA. It inhibits the synthesis of high mol.wt. Protein induced by IBA. The above features suggest that cytokinin inhibits IBA mediated root initiation by modulating the microtubular organization by way of inhibiting high molecular wt. proteins that are needed for root primordial organization. Probably this is the only example in plant system which explains the molecular events that lead to the initiation of root formation and the inhibition of it in response to combination of phytohormonal treatment. A model has been given to follow the events for new root formation in response to IBA and BAP is given.
A Model to Understand Structural Changes during Morphogenesis:
From the earlier discussions it is clear that the temporal and quantitative regulation of gene activity through transcription and translation ultimately determines structural changes during differentiation. Though not much is known about the molecular events that lead to ultra structural changes during development, it is well known that the fertilized egg in the embryo sac always undergoes a polar division as if it is predetermined and produces cells which develop into radicle and plumule. Such behavior of cells during the early part of its development is known to be a function of intracellular compartmentalization regulated by certain intrinsic interactions of nucleocytoplasmic components.
Development of umbrella shaped reproductive gametangial structures in Acetabularia, development of hold fasts in cladophora and rhizoidal formation in the zygotic development of Fucus, are some of the examples which have been used in understanding ultra structural changes during organogenesis.
The developing zygote is Fucus first produces a rhizoidal process a rhizoidal process at the region of contact with the substratum. If such spherical zygotes are subjected to unilateral illumination from one side, rhizoidal structures develop at the opposite pole. If the direction of illumination on the zygote is changed at short intervals of time, the end at which the rhizoid develops is determined by the last treatment. The time required for such polarity fixation is about 10-16 hours of treatment, beyond that the polarity of rhizoidal development remains unchanged.
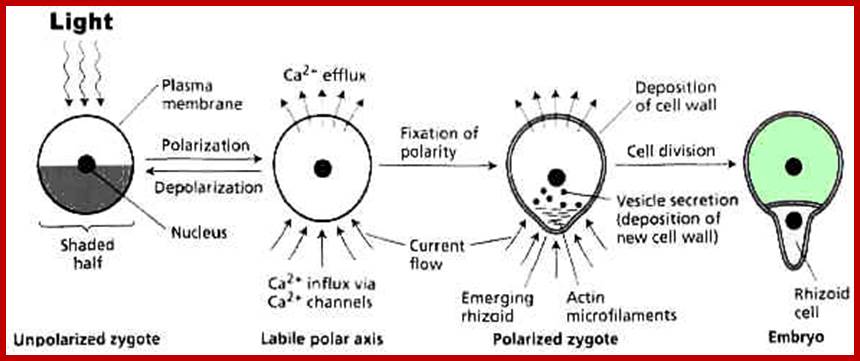
A summary of the events involved in the establishment of polarity in zygotes of the brown alga Fucus. (A) The zygote is polarized by an asymmetric stimulus from the environment, such as unilateral light. (B) A current flows (charged calcium ions move) through the polarized but still spherical zygote at the site at which the rhizoid will emerge, driven by Ca2+ uptake in the shaded half of the cell, from which the rhizoid will emerge. The current (Ca2+) flows out on the opposite side. (C) Cell polarity becomes fixed when actin microfilaments assemble at the site of rhizoid emergence and a cell wall is assembled around the zygote. The cell wall completely surrounds the zygote, but its composition differs in the rhizoid and thallus halves. Vesicles containing sulfated polysaccharides are transported by actin filaments to the plasma membrane and deposited in the cell wall only at the site of rhizoid emergence. (D) Finally, the zygote divides, and the rhizoid cell grows at its tip.www. www.5e.plantphys.net
If such programmed cells are studied under electron microscope, it is revealing to find a large number of osmophilic bodies some unknown granular materials, dense fibrillar structures, ribosomes and mitochondria congregated at the region of rhizoidal formation. Interestingly, the perinuclear region facing the rhizoidal pole shows polarized finger shaped projections indicating the flow of informational materials from the nucleus in the direction of rhizoidal pole.
The above observations suggest that the duration between the time of induction by light and the time at which rhizoids develop is the most crucial period at which various molecular and structural events fix the polarity. In fact, these events are the basis for differentiation. Furthermore, if zygotes, which re subjected photo inductive treatment, are treated with inhibitors of protein synthesis like CHI for above 9-10 hours, rhizoidal formation is not inhibited. This clearly suggests that the denovo protein synthesis is not required for polarity fixation. The polarity fixation is achieved with whatever structures or components available in the cell. Instead if such zygote is provided with cytochalasin B or colchicine or both for the same period of time, i.e. 9-10 hours, the polarity of rhizoidal formation is totally inhibited and it remains labile. Cytochalasin B and colchicine are known for disrupting the formation of microfilaments and microtubules respectively. Transmission electron microscopic studies of polarized zygotes of Pelvetia fastigata reveal cortical clearing. in the region of rhizoidal formation. Bundles of microfilaments and microtubules are found oriented in line with rhizoidal pole. The above studies clearly suggest that the polarity fixation is due to the organization and orientation of microtubules and microfilaments. The intrinsic components that lead to the organization of such cytoskeleton are not yet clear. Furthermore, the plane of cell division is also controlled by the orientation of mitotic apparatus.
It is very important to know more about microtubules and micro filaments and their role in organogenesis (refer chapter proteins). The assembly of tubulin units into microtubules and their orientation within the cell is determined by the position of nucleating centers and the availability of polymerization factors like Tau, Maps, and GTP & ATP. In a developing system, the assembly and orientation of microtubules and microfilaments are under close spatial and temporal regulation. In recent years, the role of microtubules and microfilaments in hormone induced growth and development is attracting greater attention.
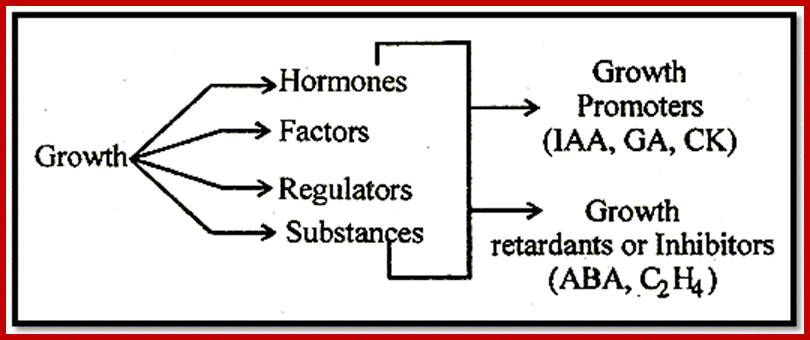
Morphogenesis involves growth and differentiation. Growth generally refers to the enlargement of the cells or the production of a group of cells of similar kind. In contrast to growth, differentiation involves the development of a cell or an organ which is structurally and functionally different from the cell it originates. In the development of a plant both growth and differentiation, go hand in hand. Plants like animals start their development from an unicellular structure, which by a series of pre-determined cell divisions produces a variety of cell structures and organs. Except for a few cell types such s sieve tubes among phloem elements, almost all living cells of all kinds are totipotent and if proper nutritional conditions are given they are capable of giving rise to a complete plant. This developmental potential is inherent in their genetic make-up. In recent years, however, it is becoming clear that the differential expression of the genetic potential is controlled by plant growth substances that are produced in the system itself. It is their interaction with the genetic apparatus which results in the full expression of the phenotype of the plant.
Supplement material from my thesis
Plant growth regulating substances- historical review:
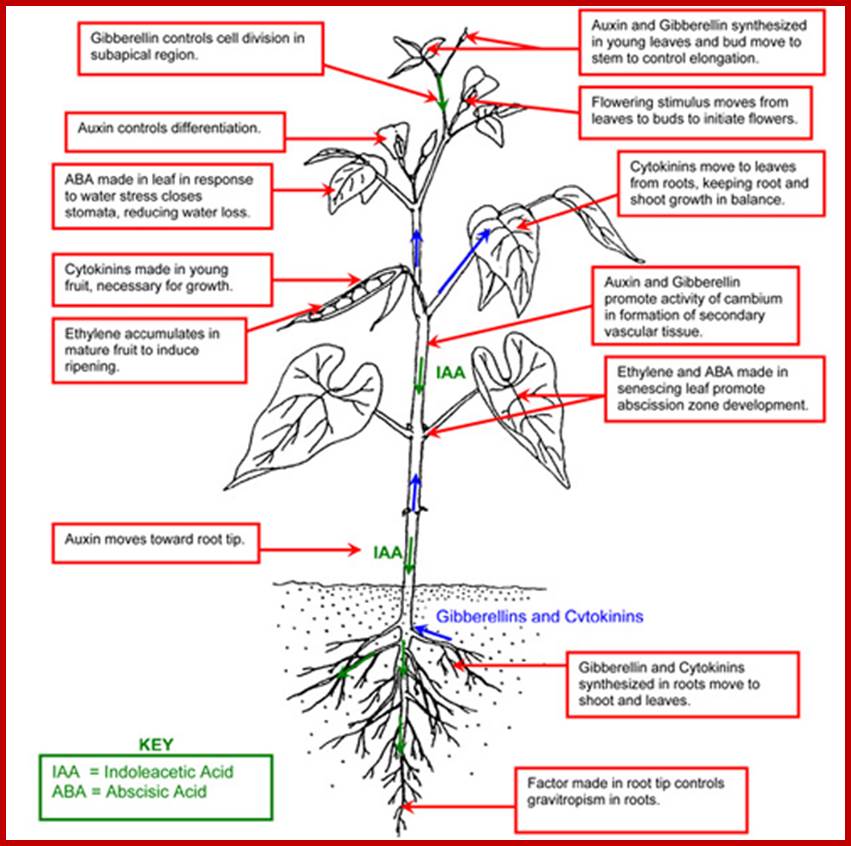
The existence of a plant growth substance was first perceived by Charles Darwin [1], who in his own elegant way, described how plants perceive the stimulus of light and respond to phototropic growth. Since then, botanists have made great strides in unraveling the mystery of the diffusible substance, which is responsible for growth. Kogl and Haagen Smit isolated and identified the growth promoting substance and coined the name “hetero auxin” or as it is known today, indole-3-ylacetic acid (IAA) [2,3]. This gave a great impetus to the plant scientists all over the world, and the search culminated in a series of discoveries of plant growth substances, such as Gibberellin B (GAB) by Yabuta and Sumuki (1938), Cytokinin by Miller and Skoog (1955), and Abscisic acid (ABA) by Robinson and Wareing (1964). Simultaneously a host of synthetic growth promoting as well as growth inhibiting substances were also made available to plant scientists. This led to intensive investigations on the effect of the various plant hormones on plants. Various hormones, elicited a wide array of responses in different kinds of tissues. It was soon realized that the physiological response to any given growth substance depends, in the first instance, on the type of cell receiving the stimulus. For example, a developing leaf cell, in a barley plant, will respond to GA3 by elongating, whereas an aleurone cell, from the same plant, will respond by producing a-amylase. The specificity of response is built into the cell by its previous developmental programme. It is curious to note that the highly specific animal hormones affecting specific cells have no counterpart in plants. In general, it can be stated that the auxin is known as growth promoting substance, but the same substance is also capable of eliciting responses such as, apical dominance, root initiation, prevention of leaf abscission, fruit setting and so on. While Gibberellic acid is known to be effective in promoting growth, induction of a-amylase, bolting and flowering, Cytokinin is considered as the hormone that controls cytokinesis, a part of cell division. Abscisic acid is known to act as a growth inhibitor, controlling processes such as bud dormancy and seed dormancy. Ethylene, recognized as a hormone recently, shows bizarre effects. In the development of the plant body, it is the interaction between the various hormones that controls and regulates the process of morphogenesis.
http://www.biosci.ohio-state.edu/
Hormonal Interplay:
Although Haberlandt (1913) considered the tissue and organ culture in sterile conditions as a theoretical possibility, it was realized as a practical proposition for research by two independent workers, Kottle (1992) in Germany and Robbins (1922) in America. The advent of tissue culture techniques opened up a new vista in the studies of hormonal interactions in morphogenesis.
When plant growth substances are supplied to plant tissues exogenously, each of them elicits unique responses. However, many responses are overlapping. But if two or more different hormones are supplied in known concentrations, in many cases synergistic or antagonistic responses are exhibited and this effect is referred to as hormonal interaction.
Studies on tobacco pith callus tissue have revealed that the relative concentrations of hormones control the expression of callus either into roots or shoots. Low, high and intermediate ratios between auxin and cytokinin induce roots, shoots and callus respectively [4]. Auxin-cytokinin-induced shot formation in the callus is inhibited by GA3; however GA3 enhances the growth of the callus. The inhibitory effect of GA3 can be alleviated by the addition of ABA, which by itself is a weak inhibitor of callus growth [ 5,6 ].
The form of shoots developed from callus is dependent on GA3-cytokinin ratio. The higher ratios induce the formation of tall, spindly shoots with narrow leaves, whereas lower ratios produce dwarf shoots with rounded leaves [7].
Specific hormones are found to influence the relative levels of other hormones and this is very well substantiated in the culture system, where auxin-cytokinin induced callus growth is reduced by certain culture conditions, which also inhibit the biosynthesis of GA [6]. Thus, the interactions of hormones appear to be very complex.
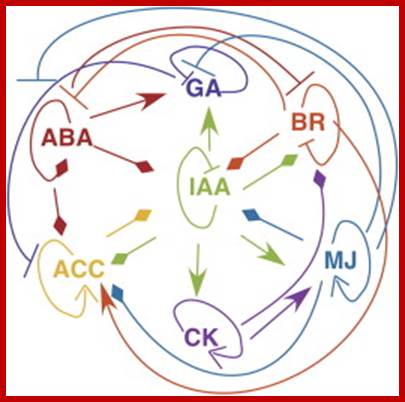
This complexity is further compounded by the recent observations, in which, it has been shown that each hormone can influence the biosynthesis, degradation, conjugation or transport of one or the other hormones. The endogenous level of IAA in a plant tissue is enhanced by the exogenous application of GA or Kinetin. But, ethylene, which is known to be induced by excess auxin, reduces the level of auxin itself. Again, auxin levels can be increased by the addition of cytokinin. This complex relationship appears to be very fascinating, because of the findings in which cytokinins are known to bring down the levels of ABA, through the increased biosynthesis of GA. But ethylene promotes the synthesis of ABA, which in turn enhances the levels of ethylene. The close relationship between ethylene and auxin provides an excellent feed back control mechanism. Letham et al. (1978) have illustrated the complex interaction between plant growth substances as indicated in the diagram (Fig. 1) [8]. Further investigations into hormonal interactions at molecular level, will have far reaching impact in understanding morphogenesis at the molecular and sub cellular levels.
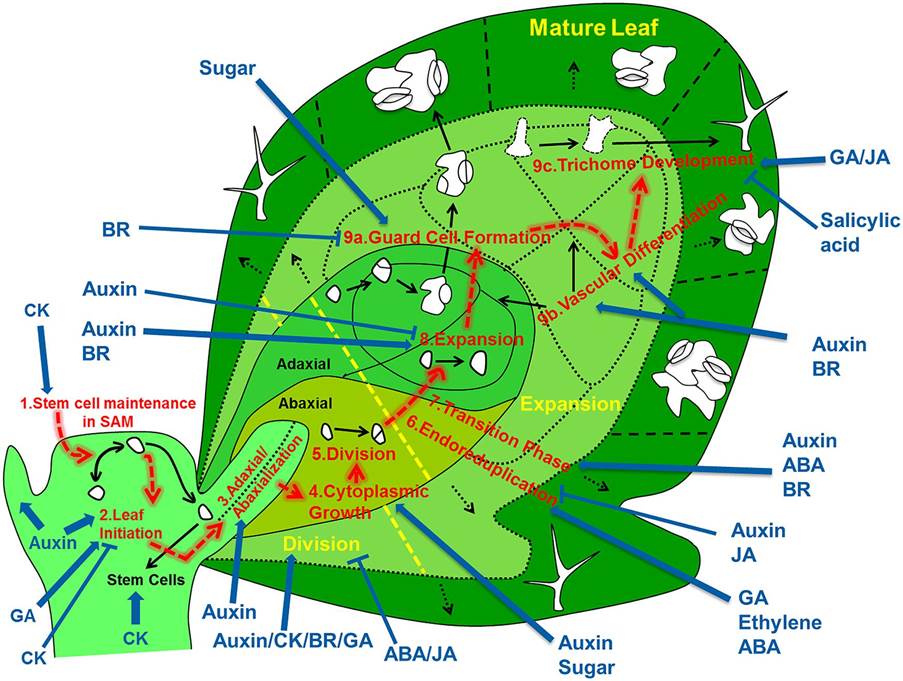
Overview of the regulatory processes that determine the development of a leaf. The cells that form the leaf originate from the stem cell niche at the shoot apical meristem. As a first step in their development, cells need to loose stem cell identity (1). A leaf primordium is initiated in groups of cells that migrate into the lateral regions of the SAM (2), which further acquires upper (adaxial) and lower (abaxial) sides through leaf-polarity control (3). Afterward, the transformation of the small leaf primordium to a mature leaf is controlled by at least six distinct processes: cytoplasmic growth (4), cell division (5), endoreduplication (6), transition between division and expansion (7), cell expansion (8) and cell differentiation (9) into stomata (9a), vascular tissue (9b), and trichomes (9c). Most of these processes are tightly controlled by different signaling molecules, including phytohormones. The developmental path of cells is indicated with red arrows, key regulatory processes are numbered and indicated and regulation of these processes by phytohormones/sugar is shown by blue arrows (pointed and T shaped arrows indicate positive and negative regulation, respectively). http://dev.biologists.org/
Ultra structural changes during differentiation: Microtubules, Microfilaments in cell polarity and organogenesis:
The temporal and quantitative changes in the regulation of gene activity form the basis for the ultra structural changes determining the process of cell development.
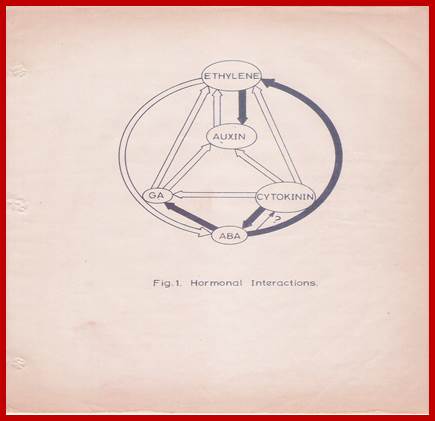
Hormonal Interactions; GrkRaj
![]()
![]() Fig.1: Hormonal interactions which
affect hormonal levels in plant tissues. Open arrow from A B indicates
hormone A causes increase in the levels of B. Closed arrow from A B
indicates that hormone A decreases the levels of B. differentiation.
Fig.1: Hormonal interactions which
affect hormonal levels in plant tissues. Open arrow from A B indicates
hormone A causes increase in the levels of B. Closed arrow from A B
indicates that hormone A decreases the levels of B. differentiation.
The molecular events leading to such ultra structural organization are not very clear. For example, it is not clear how and why a fertilized egg in the embryo sac, always undergoes a polarized division in such a way, that one of the two cells, equal or unequal, develops into radicle and the other into plumule. This polar behavior of the cells at the earliest part of their development is the function of intracellular compartmentalization, regulated and governed by certain intrinsic interactions of nucleo cytoplasmic components, whose expression in turn is controlled by the inherent genome.
Polar growth like cap (reproductive organ) formation in Acetabularia, rhizoidal development in Fucus, hold-fast development in Cladophora are some of the best studied systems in the plant kingdom. In the case of zygote development in Fucus, if the spherical zygote is subjected to unidirectional light treatment, the rhizoid develops at the shaded side of the cell. If such a programmed zygote is subjected to electron microscopic investigation, it is observed that in the region of rhizoid formation, large number of osmophilic bodies and undefined extracellular materials accumulate. The peri nuclear region showed highly polarized finger shaped projections towards the site of rhizoid initiation. Along with these charges, heavy concentration of mitochondria, ribosomes and dense fibrillar vesicles are also found. These structural changes represent an expression of a fixed axis for rhizoidal growth [9]. The time taken for such polarity fixation has been determined to be 10-16 hrs. During this period, if the cells are treated with cycloheximide (CHI) for 9-15 hrs after fertilization, they still show a fixed polar axis. However, if cytochalasin-B, which is known to disrupt the microfilaments, is present during polarity fixation, the polar axis remains labile and axis establishment is prevented [10].
The role of cytochalasin-B and colchicines, in lactin Con-A induced “cap” formation in animal cells, has been reviewed by Edelman (1976) and Nicolson (1976) [11,12]. The authors have explained how the microtubules and microfilaments control the mobility, stabilization and topographical distribution of cell membrane and surface components during lectin induced “cap” formation. The cross linking of membrane receptors embedded in the plasma membrane, to a variety of fluorescent labeled polyvalent ligands such as antibodies or lectins lead to form patches and clusters which ultimately culminates in the “cap” formation at one end of the cell. Similar to “cap” formation in animal cells, “cortical clearing” in the region of rhizoidal emergence is observed in zygotes of the plant Pelvetia fastigata [13]. In the same system, Peng et al. (1976) have shown the deposition of new membrane patches (30 um) covering the rhizoidal region at the time of cortical clearing [14]. These studies clearly suggest that microtubules and microfilaments play a significant role in cell polarity, which is a prerequisite for organogenesis. Ever since, the discovery of microtubular components in few plant cells by Lederbetter and Porter (1963) [15] the presence of such structures, in most of the plants, has been established.
Basic components of the microtubules are not known to be tubulin monomers, which exist in the form of heterodimers, consisting of α and β subunits, of molecular weight 110,000- 120,000 daltons [16]. Several proteins are associated with microtubules, of which the proteins known as TAU and microtubule associated proteins (MAPS) appear to be important, for they take part in assembly and disassembly of microtubules [17,18,19]. In addition to the above components, the presence of nucleating centers or organizing centers within the cells, account for an ordered or controlled assembly of microtubules [20,21]. In a developing system, microtubule assembly is under close spatial and temporal regulation. The nucleating centers function in controlling initiation, orientation, directionality and patternization of microtubular polymerization. Recently attempts have been made to isolate microtubules and study their organization in plants [22,23]. The cross reaction of antibodies, raised against porcine brain tubulin, with the plant protein, strongly suggests the similarity of plant tubulin with animal tubulin, at least at the antigenic level. Considerable attention has been given to relate the orientation of microtubules during plant hormone induced growth. Hormone induced growth in Vigna angularis, hypocotyl of Lactuca sativa and coleoptile segments of Triticum vulgare, is inhibited by colchicines, urea and vineblastin sulfate [24-26] and this inhibitory effect is attributed to microtubular disruption [27]. Similarly, the inhibition of protoplasmic streaming in Avena, and growth inhibition in Zea mays root by cytochalasin-B are again attributed to the role of microfilaments and microtubules [28,29]. Electron microscopic studies on the deposition of cellulose in cell walls during secondary growth and movement of secretory vesicles are shown to be directed by microtubules [30]. However, the available information on plant hormone induced changes in the microtubular assembly, synthesis and their role in morphogenesis is scanty.
Plant hormones and Nucleic acid metabolism:
Plant growth regulating substances; very often, exhibit dramatic effects on growth and development of plants. The molecular mechanisms which control such processes involve changes in nucleic acid and protein metabolism. DNA replication, transcription and translation are the three major molecular events involved in growth and differentiation. An understanding, as to how plant hormones control such molecular mechanisms, as to how plant hormones control such molecular mechanisms, is very pertinent to interpret morphogenesis at the molecular level. In spite of the enormity and complexity of the problem involved, considerable Progress has been made in this field.
DNA metabolism:
A majority of investigations reveal that hormone induced cell elongation does not require DNA synthesis [31,32]. However, Wardell (1975) has reported that auxin, in flowering tobacco plants, enhances DNA synthesis within an hour of treatment. On the contrary, in pea root cortical cell cultures [33], Libbenga and Torrey (1973) have shown that enhanced DNA synthesis is possible, only in the presence of auxin and kinetin together, but not with auxin alone [33a].
In long term experiments, most of the growth promoting substances enhances DNA synthesis which is always accompanied by cell division [34]. In cucumber hypocotyls, GA3 – promoted growth is fluoro-deoxyuridine (FUDR) sensitive. This increase in DNA synthesis, in response to GA3 is due to the increased chloroplast proliferation, which is prevented by chloramphenicol. The above experiments suggest that GA3 has some preferential effect on chloroplast biogenesis [35, 36].
On the other hand, hormones such as ethylene and Abscissic acid inhibit DNA synthesis. This correlates well with their growth inhibitory effects in vivo [37-39]. However, it is not clear whether this effect is direct or indirect.
Although, cytokinin is known to induce cell divisions, it does not affect DNA synthesis by itself as a prelude to cytokinesis. However, it promotes DNA synthesis in the presence of auxin [40]. Hence, it is suggested that auxin has a permissive role in DNA synthesis, while kinetin stimulates it. This is further exemplified in an experiment, where hormone depleted and aged tobacco pith explants, could be induced to undergo mitosis without the accompanying DNA replication by the addition of cytokinin. This suggests that cytokinin perhaps regulates cell division, by controlling the synthesis of specific proteins for mitosis rather than acting directly on DNA replication [41,42].
DNA sequence complexity:
In higher plants, it is estimated that the amount of DNA present per cell ranges from 1pg – 100 pg and this amount of DNA codes for more that 106 different proteins. The rough estimates indicate that there are 50,000 or more structural genes which are expressed at one time or the other during the life of the plant [43]. The number of structural genes operating in different plant structures like root, stem, leaves and flowers may vary. On the basis of hybridization studies between polysomal RNA and 3H-labelled single copy DNA, it has been estimated that there are about 2700 diverse structural gene transcripts in the tobacco leaf cells [44]. DNA, in higher plants, consists of three base sequence classes i.e., highly repeated, moderately repeated and unique. Unique sequences are believed to code for mRNA sequences [45]. These sequences can be detected, characterized and quantified by studying the reassociation kinetics of denaturated and sheared DAN. Highly repeated sequences reassociate rapidly at low cot values ( cot = concentration of DNA in mole nucleotide per liter X time in seconds ) and unique sequences reassociate slowly at high cot values [45]. Studies on changes in DNA sequence complexities in response to phytohormones in plants are few. Nevertheless, reannealing studies of DNA isolated from the control and auxin treated artichoke tubers; suggest that auxin causes an apparent loss in the rapidly reannealing fraction of DNA, but leads to some dramatic changes in the cot values for other fractions [46]. On the contrary, GA3 has no effect on the DNA isolated from the roots of Cucumber but DNA from the treated shoots and leaves of the same plants, exhibit a nine-fold increase in the intermediate reassociation fractions. According to Britten and Davidson (1969) such sequences may have a regulatory role [47]. In cymbidium protocorm tissue, while auxin causes an increase in AT-rich DNA, GA3 promotes an increase in GC-rich DNA [48]. Similar studies have been carried out in various plants such as wheat embryos, pea epicotyls etc. [49]
However, the above studies do not indicate whether, phytohormone induced changes in DNA population is due to primary or secondary effects of the hormones.
Phytohormones and transcriptional activity:
Protein synthesis is a crucial event in growth and differentiation, and therefore the effect of phytohormones on transcription and processing of different kinds of RNA would determine the process of morphogenesis.
Auxin-a mediator/acceptor in protein and RNA synthesis:
The mechanistic aspects of auxin function appear to be very complex. Previous investigations have suggested that there may be one or two cellular sites at which auxin may act and elicit its responses on growth and differentiation. One such site is found to be located in the plasma-membrane and the other may be a non-plasma membrane site located either in the cytoplasm or nucleus.
Auxin-mediated early cell enlargement is known to function through the activation of membrane bound factors [50-52]. The early growth is insensitive to both cycloheximide and actinomycin D; hence neither RNA nor protein synthesis is required for this function. However, the second phase of growth requires both RNA and protein synthesis. The auxin-induced mechanism of early growth is speculated as due to the cell-wall loosening effect caused by the secreted protons, which are pumped out by the auxin-activated membrane bound factors [53]. The existence of soluble auxin-binding proteins of molecular weight 3,15,000 and 10,000 daltons have been reported in dwarf bean seedlings and soybean cotyledons respectively [54,55]. It is not clear, whether these auxin binding proteins have any role in transcription. Hardin et al. (1972) [56-58] have demonstrated that in soybean hypocotyls and onion stems, auxin treatment results in the release of a plasma membrane factor into the cytosol, which stimulates a-Amanitin sensitive RNA polymerase activity. However Mathyse and Philips (1969) have shown the existence of auxin-receptor complex in the nuclei and this factor is responsible for the enhanced RNA synthesis. Auxin-receptor protein has been isolated from coconut nuclei and shown to have a molecular weight of 10,000 daltons. It binds to the auxin non-covalently at a molar ratio of 2:1. In the presence of transcribing system, consisting of a-amanitin sensitive RNA polymerase (presumably RNA polymerase II), native DNA, and an initiation factor, the auxin binding factors stimulate RNA synthesis 2-3 fold, only in the presence of auxin. Although, these factors are not well characterized, this constitutes a good evidence for the existence of auxin-receptor proteins.
Using changes in melting point profiles and bathochromic shifts in DNA and chromatin, Fellenberg (1971) and Bamberger (1971) have claimed that auxin interacts with DNA and chromatin directly, there by facilitating transcription [62,63]. However, these observations turned out to be due to changes in the PH of the medium, rather than to the direct effects [64].
While the studies on auxin-histone interactions have not yielded any significant information, non-histone proteins are now considered to be the most likely candidates influencing specific gene transcription. Non-histone proteins are heterogenous and include a number of enzymes and structural proteins. They are species-specific and tissue-specific. Their phosphorylation pattern changes with the different states of gene activity [65,66]. Such an involvement is very well exemplified in the studies involving progesterone and estrogen receptor complexes with the chromatin of target cells in different animal systems.
In plants, the existence of zone-specific non-histone proteins is known in soybean hypocotyls [68]. Recently, 2,4-dichlorophenoxy acetic acid (2,4-D) induced changes in the phosphorylation of nuclear proteins has been shown in soybean, but the correlation between auxin induced RNA synthesis and the phosphorylation pattern ahs not been established unequivocally [69].
Tissere et al. (1975) have isolated factors from the acidic fraction of the chromatin proteins from the lentil roots, referred to as a, b, g and d factors. On auxin treatment, the a, b and d factors remain unchanged but the g factor increases two-fold. While the g factor regulates RNA polymerase I , d affects polymerase I and II. Hence these factors are known to regulate the activity of RNA polymerases without enhancing the synthesis of the enzyme [70].
Generally, auxins induce RNA synthesis. Silber and Skoog (1953) for the first time correlated auxin-induced cell enlargement with enhanced RNA synthesis [71]. Since then, considerable work has been carried out on the auxin induced changes in the levels of various RNA species.
Auxin has been reported to preferentially increase rRNA content in pea epicotyls and soybean hypocotyls [72]. But, it has also been shown that the enhancement in RNA synthesis occurs well after auxin has effected growth. It has been found that 5-fluorouracil [5-FU] does not prevent cell enlargement in soybean hypocotyls and artichoke tuber, but auxin induced growth in oat coleoptile and soybean hypocotyls is inhibited by actinomycin D. The above observations suggest that auxin induced growth in the earlier stages does not require RNA synthesis, but requires the synthesis of messenger RNA later [73,74].
Investigations, using polysome content as a measure of quantification of mRNA synthesis have shown that auxin induced polysome levels are actinomycin D sensitive, but 5-FU insensitive. This indicates that the synthesis of new ribosomes is not necessary for the increase in the levels of polysome [75-78]. Verma et al. (1975) using in vitro cell free protein synthesizing system and cellulase specific antibody, have shown that the auxin induces the synthesis of cellulase-specific RNA [79]. However, increase in other m RNA populations is not ruled out.
It is not clear, whether, the auxin induced messenger RNA is synthesized as a precursor heterogenous RNA (hnRNA) in plants. In short-term labeling experiments (45 minutes), auxin enhances the labeled precursor incorporation, preferentially into the nuclear fraction. Long term labeling experiments; however indicate that incorporation into both nuclear and cytosolic fractions is enhanced [80]. Recent studies with Petroselium sativum (parsley), have demonstrated that most of the poly (A)-RNA is synthesized as hnRNA. This is established by DNA-RNA hybridization-kinetic studies [81].
Attempts have also been made to use Escherichia coli RNA polymerase core enzyme, to measure transcription of DNA/chromatin, as an index of differential gene expression chromatin, isolated from auxin treated soybean seedlings has been found to code for synthesis of many fractions of RNA species, of which the synthesis of TB-RNA, several fractions of hetero disperse RNA AND 4S-5S RNA are much more than the control. This chromatin preparation enhances labeled amino acid incorporation into proteins by 2-3 fold in coupled transcription-translation assays [82,83]. Similar studies involving the plant tissues have clearly indicated that auxin can influence polymerase activity, as well as template activity.
GA binding/mediator protein and RNA synthesis:
The presence of GA3 – binding protein in pea stems, epicotyls and other plants has been reported. The approximate molecular weight of the GA3 – binding proteins, extracted from dwarf epicotyls of pea plant, has been determined to be 5, 00,000 and 60,000. Both the fractions bind GA3 noncovalently and easily exchange with non-labeled GA3.
Not much is known about the effects of GA3 binding proteins on transcriptional events. Isolated pea nuclei do not respond to exogenously added GA3 but, if the nuclei are isolated after GA3 treatment, transcription is enhanced [86]. Attempts to localize GA3 – binding sites, in cells by autoradiography or by cell fractionation, have yielded limited results [87]. GA7 has been shown to interact with DNA containing higher AT than GC content. It is also reported that GA7 interacts With DNA isolated from a number of plant sources, as well as some phages, but it does not interact with either animal or bacterial DNA. This specificity of GA7 interaction has not been explained. In the presence of DNA Ligase, GA7 causes the formation of covalently linked loops in cucumber DNA, but cytokinins and auxins do not have any effect. Recently, it has been reported that at low concentrations of ethidium bromide, GA4, GA7, or GA3, show synergistic effects on elongation in cucumber hypocotyls, perhaps by exposing binding sites in DNA and thus making gibberellic acid more effective.
Studies on the effect of GA3 on transcription of either isolated chromatin or isolated nuclei from pear or cucumber show, that the increase in RAN synthesis is due to increased RNA polymerase, as well as due to the increased template availability [90]. A wide variety of tissues such as barley leaf segments, clover seedlings, lentil epicotyls, potato buds, cucumber hypocotyls and sugar beet have been used to study GA3’s effect on RNA synthesis. A significant amount of information on the effect of GA3 on RNA synthesis comes from the studies with barley aleurone cells. GA3 causes a selective increase in m RNA for a-amylase, which on release to the endosperm facilitates the mobilization of carbohydrate source for the growing embryo. If actinomycin-D is added along with GA3, the increase in a-amylase synthesis is inhibited [91]. The labeling of poly (A)–RNA, specific for a-amylase, shows an increase after GA3 treatment with a lag period of 3-4 hrs. This correlates well with the lag period of a-amylase synthesis observed in vivo. However, Higgins et al (1976) and Carlson (1977) have suggested that GA3 activates the preexisting a-amylase m RNA by post transcriptional process into an active translatable form of m RNA but, Rodaway et al. (1978) have considered this possibility as very unlikely similar effects of GA3 on poly (A)-RNA synthesis have been established in etiolated maize seedlings, but their translational products have not been characterized.
Cytokinin- receptor protein and RNA synthesis:
The presence of cytokinin-receptor protein has been indicated by the work of Mathyse et al. (1969) [97]. The cytokinin-protein complex enhances RNA synthesis in vitro in presence of pea bud chromatin or DNA as template and E. coli or calf thymus, kinetin-receptor does not stimulate RNA synthesis. Similar studies using nuclei-rich preparations from soybean, tobacco callus and pea buds, have shown that the cytokinins enhance [14 C] –ATP incorporation into TCA precipitable and KOH digestible RNA fractions by 40-100% within 5-10 minutes after treatment. To the phytohormone-starved tobacco pith callus, the addition of cytokinin alone does not cause any major charges in RNA metabolism, but cytokinin with auxin treatment enhances [32 p] – orthophosphate incorporation into polysomal RNA within 3 hrs of treatment [98,99]. On the contrary, in the hypocotyls of soybean, even at early stages after treatment, the auxin induced incorporation of [14 C] –uridine into RNA is inhibited by kinetin. After 6 hrs of incubation with cytokinin and auxin together, the inhibition of labeled precursor incorporation into rRNA and tRNA is 90% and TB-RNA 30-50%. However, the labeling into D-RNA is not affected [ 100-103 ].
Cytokinin-mediated increase in the specific isoacceptor leucyl-tRNA species has been detected in cotyledons and hypocotyls of beans [ 104, 105 ]. This could be the result of and effect of direct synthesis or on post-transcriptional modifications. In general, it seems that the occurrence of cytokinins in tRNA is not longer viewed as necessary for eliciting the cytokinin response [106-108].
Ethylene and RNA metabolism:
Although no coherent information is available regarding ethylene binding to receptor proteins, transcriptional studies with isolated chromatin from ethylene treated tissue, show qualitative changes in the RNA species synthesized. There is a change in the nearest neighbor frequency of the RNA synthesized, thus indicating transcription of new template in response to ethylene [37].
Generally ethylene is known to induce abscission and fruit ripening in plants. During abscission, ethylene enhances the synthesis of cellulase and pectinase, which are responsible for the breakdown of the cell wall and pectin [109, 110]. In cotton, Coleus and bean plants, ethylene induced abscission can be prevented by actinomycin D. Furthermore, using 5-FU as an inhibitor of rRNA synthesis, investigations have demonstrated that the ethylene mediated abscission involves induction of specific mRNA, but the products have not yet characterized.
Similar results have been obtained in the ethylene induced ripening of Pyrus mallus fruits [111]. In ripening tomato fruits, ethylene brings about changes in the relative amounts of iso-accepting species of leucyl-, lysyl-, methionyl- and tyrosinyl-tRNAs.
ABA and RNA metabolism:
ABA affects a number of physiological processes, such as dormancy, leaf senescence, abscission, seed germination and flowering, and also antagonizes the effects of other plant hormones, in a number of plant tissues. It is not clear, whether such physiological responses to ABA are brought about through mediator molecules. However, various reports indicate that the effect of ABA is on RNA – turnover and translational process.
ABA has been shown to inhabit the transcriptional capacity of isolated chromatin [112]. This inhibitory effect is ascribed to the effect of ABA on polymerase activity [113, 114 ]. In Fraxinus embryos [115], ABA inhibits labeled precursor incorporation into RNA, and reduces polysome formation, but protein synthesis is not affected. The above results have been interpreted to mean that ABA prevents the synthesis of mRNA and other RNA species, thereby affecting growth.
ABA, in pear embryo and lentil roots, causes a preferential increase in UMP rich RNA and a substantial decrease in GMP, but there is not change in AMP, CMP contents of RNA (115 a). This observation is significant in view of the recent observation, that translation control RNA (tcRNA), which controls translation of certain mRNAs has very high (50%) content of UMP nucleotides [116]. Additional support to the above observation comes from the findings that ABA treated germinating seedlings contain UMP rich (40-50%) RNA species [117]. It is not clear whether this high level of UMP rich RNA is due to new synthesis or preferential degradation of the other RNA species.
ABA also inhibits GA3 - promoted a-amylase synthesis, in barley aleurone cells. Some investigators have shown that ABA inhibits [14 C] – uridine incorporation into polydisperse RNA [92, 118]. There are also reports that ABA does not inhibit the synthesis of any species of RNA [119]. In this context, the observation about certain short chain fatty acids (of C5 and C9 length) at a concentration of 10-4 M, inhibiting GA3 – induced amylolysis in barley endosperm is interesting. Such short chain nonionic fatty acids have been detected in many germinating seeds such as oat and fenugreek [120].
Phytohormones and post-transcriptional events:
Perichromatin and Precursor RNAs:
Most of the RNA species, transcribed within the nucleus, are in the form of larger molecules and these are processed and transported to the cytoplasm across the nuclear membrane pore complex [121]. The existence of extra chromosomal and extra nucleolar ribonucleoprotein structures called perichromatin granules (presumably precursors of mRNAs) in rat liver nuclei and plant cells, has been reported as early as 1969 [122]. The transport of such particles across the membrane pore complex has been speculated. Active movement of such chromatoid body (containing RNA) across the pore complex, during rat spermatogenesis is every well documented [123]. In plants, synthesis of large precursor RNAs (hnRNA) and their processing into the mature forms have been reported [43,124,125].
Poly (A) RNA with ‘caps’
During the processing of hnRNA, additional structures such as the “cap” at the 5’ end poly (A) chain at the 3’ end are tagged to the mRNA. Addition of poly (A) segments of 50-200 nucleotides, at the 3’ end of the hnRNA or mRNA is catalyzed by poly (A) polymerase [126]. The existence of such polyadenylated mRNA has been demonstrated in the nuclei and cytoplasm of plant cells [93,127,125]. However, not all mRNAs of RuDP carboxylase in chloroplasts lack such mRNA (?), it is one such example [128].
Capping at the 5’ end of the mRNA involves the addition of 7-methylguanosine, which is linked by a 5’-o-P-o-P-o-P-o-5’ triphosphate bridge to a 2’-0-methylated Adenine [129,130]. The “cap” structure is implicated in influencing the processing of pre-mRNA, initiation of translation, transport of mRNA and stability of the same [131, 132]. However, “cap” is not required for the translation of all mRNAs [133]. Recent reports suggest that the prokaryotic mRNAs, which are “capped” in vitro, translate very efficiently in cell-free eukaryotic protein synthesizing system [134]. Sonenberg et al. (1979) have isolated a “cap” binding protein whose molecular weight is 24,000. This protein stimulates translation of capped mRNAs in an in vitro system derived from HeLa cell extracts, under conditions that do not increase translation of non-capped mRNAs from satellite necrosis virus [134a]. In plants, there are not many reports about “cap” structures in mRNAs. However, addition of 7-methyl guanosine 5’-monophosphate (m 7G5P) to the French bean seed mRNA – programmed cell free protein synthesizing system derived from wheat germ, inhibits [35 S] - methionine incorporation into proteins by 90%, thereby suggesting that the mRNA isolated from French be an cotyledons are “capped” [135]. The role of plant growth substances in the “capping” process is not clear.
The non-coding sequences found between the first AUG codon and the “cap” at the 5’ end and the last terminator codon and the first adenine nucleotide of the poly(A) segment at the 3’ end are suggested to play an important role in initiation and termination of protein synthesis [136]. Such information is not available with plant mRNAs.
Informosomes:
The messenger RNA is transported from the nucleus to cytoplasm as m RNA protein complex (m RNP). These m RNP particles may release the m RNA for polysome formation or the m RNPs may remain in an inactive form. The latter particles are referred to as informosomes. Such informosomes are found in a number of embryonic tissues [93,137,138] and other non-embryonic structures such as stems, tubers, etiolated leaves, dry mosses, spores of ferns, Acetabularia and Dictyostelium. Such inactive m RNPs have also been reported in animal cells [145-147].
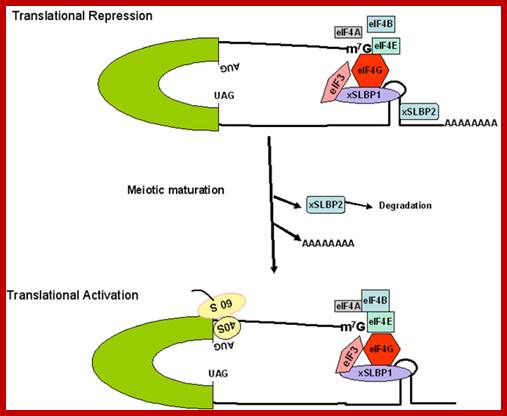
www.Bioscience.org
The m RNPs that are found in most of the organisms have poly(A) tail at 3’ end [148]. The presence of such m RNPs ensure the preservation of m RNAs during periods of dormancy and certain adverse environmental conditions such as drought, lack of optimal photoperiodic stimulus and lack of light of actinic wavelength.
Activation and inactivation of mRNAs:
The activation of preexisting dormant m RNPs to external stimuli has been reported in a variety of systems such as germinating seeds [138,159] rejuvenating moss[141], etiolated leaves exposed to light [140], ferritin synthesis in rat liver [146] and fertilization in sea urchin eggs [160]. Specific effects of plant hormones on this process have also been documented. These examples include GA3 effect in barley aleurone cells and spores of Anemia phyllitidis [93,149], IAA effect in pea epicotyls [79] and cytokinin effect in Glycine max [102,103]. In all the above mentioned cases, when external stimuli are provided, the dormant m RNPs get activated and the rate of protein synthesis is enhanced, even in the presence of actinomycin-D, or 5-FU, thereby indicating that the enhanced protein synthesis is independent of new RNA synthesis. It is not clear, whether these external stimuli or added phytohormones act directly on m RNPs or function through other mediating-factors, which may either activate ribosomal machinery or bring about the conformational changes in the secondary structure of m RNPs.

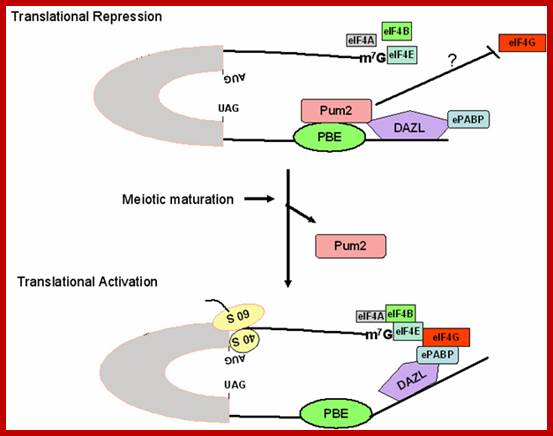
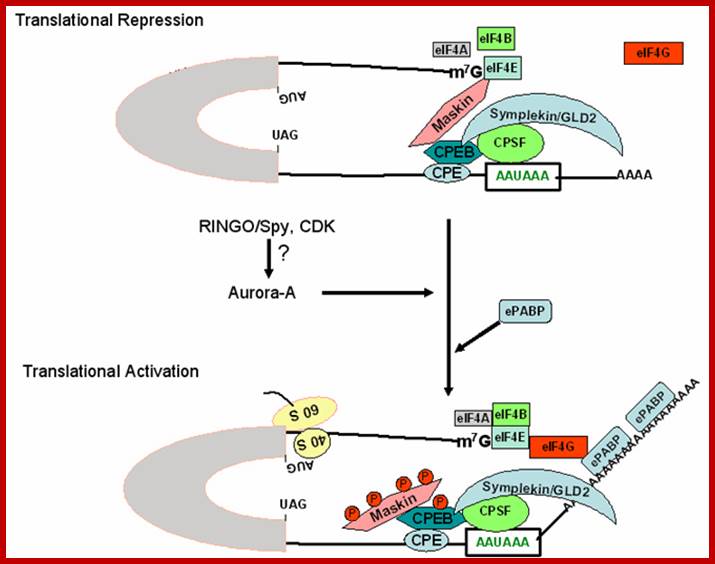
tRNAs:
Transfer RNA (tRNA) is also synthesized as a large precursor and then processed into smaller products [131]. Transport, slicing and secondary modifications such as methylation, thiolation of pre-tRNAs are some of the important controlling processes, which make available, the functional tRNAs for protein synthesis. Addition of CCA at 3’ terminal of t RNA and activation of amino-acyl tRNAs and activation of amino-acyl t RAN synthetases are other crucial steps that regulate the translation processes in Pisum sativum seedlings, GA3 is known to enhance aminoacyl t RNA synthetases by 5-8 fold [149]. In early 1960s, auxin was suspected of form a complex with t RNA thus regulating t RAN function [150]. However, it is now considered that this may not be the case [151,152].
As the genetic code is degenerate, it is thought, that multiple iso-accepting species of tRNAs may exert a regulatory role in the translation of mRNAs [153] and the relative changes in the population of such iso-accepting species of t RANs [154] and the relative changes in the population of such iso-accepting species of tRNA during different stages of development, has been recorded in plants [155,156]. The presence of cytokinins-effect may be mediated through tRNAs [106]. The occurrence of the highly active cytokinin ^6 – de (6-3 methyl-2-butenyl amino purine) adjacent to the A^ base of the anticodon triplet has been demonstrated in yeast serine- tRNA I, II and E. coli- tRNA [157]. Such cytokinin containing tRNAs have been identified in various plants and it is felt, that cytokinins may exert their effect on growth through regulating the translational process. However, recent studies [158] show that there is no correlation between the effect of hormone-induced growth and the presence of cytokinin in tRNA species. Nevertheless, there are evidences to show that cytokinins can affect the tRNA activities either by regulating the levels of tRNAs or by secondary modification of tRNA by methylation or by changing amino acyl-synthetase activity [108].
Control of protein synthesis by phytohormones:
![]() In radish cotyledons, cytokinin-enhanced protein
synthesis does not require mRNA synthesis de novo [161].
Similarly cytokinin enhances protein synthesis in soybean callus, as early as
30 minutes after treatment [102]. Klambt (1976) [99] has demonstrated that N5
(2- isopentyl) adenine stimulates cell-free protein synthesis slightly in
systems derived from tobacco pith and corn extracts. Such stimulation is
suggested as due to selective binding of cytokines to a specific protein of the
ribosome. Fox et al. (1975) [162] have identified one low and
one high affinity cytokinin binding site on wheat germ ribosomes. The binding
affinity is lost on boiling the 0.5M KCL wash or on treatment with trypsin,
suggesting that this high affinity receptor is a ribosomal protein. Such
cytokinin-binding protein from tobacco callus is associated with 4OS ribosomes
[163]. In animal systems [164] increased or reduced phosphorylation of
ribosomal protein controls the rate of protein synthesis. Cytokinin is also
thought to enhance protein synthesis through dephosphorylation of ribosomal
proteins. Tapfer et al. (1975) [165] have found that, when
cytokinin requiring soybean callus is transferred to a cytokinin deficient
medium, the decreased rate of protein synthesis is accompanied by increased
phosphorylation of some ribosomal proteins.
In radish cotyledons, cytokinin-enhanced protein
synthesis does not require mRNA synthesis de novo [161].
Similarly cytokinin enhances protein synthesis in soybean callus, as early as
30 minutes after treatment [102]. Klambt (1976) [99] has demonstrated that N5
(2- isopentyl) adenine stimulates cell-free protein synthesis slightly in
systems derived from tobacco pith and corn extracts. Such stimulation is
suggested as due to selective binding of cytokines to a specific protein of the
ribosome. Fox et al. (1975) [162] have identified one low and
one high affinity cytokinin binding site on wheat germ ribosomes. The binding
affinity is lost on boiling the 0.5M KCL wash or on treatment with trypsin,
suggesting that this high affinity receptor is a ribosomal protein. Such
cytokinin-binding protein from tobacco callus is associated with 4OS ribosomes
[163]. In animal systems [164] increased or reduced phosphorylation of
ribosomal protein controls the rate of protein synthesis. Cytokinin is also
thought to enhance protein synthesis through dephosphorylation of ribosomal
proteins. Tapfer et al. (1975) [165] have found that, when
cytokinin requiring soybean callus is transferred to a cytokinin deficient
medium, the decreased rate of protein synthesis is accompanied by increased
phosphorylation of some ribosomal proteins.
In soybean hypocotyls, a parallel situation is found where Auxin causes increased protein synthesis in a cell-free protein synthesizing system [166]. Travis et al. (1976), have proposed, that the polysome formation is dependent on ribosome activation as well as mRNA supply. In the same system they have also observed the incorporation of labeled amino acids into 3 ribosomal proteins, one of which shows several fold higher incorporation that that of the control. However, ribosomal dissociation studies, clearly indicate that these auxin induced proteins are associated with 60S ribosomal sub-units. Evidence for hormone-induced protein synthesis, independent of mRNA synthesis, is also found in Pea epicotyls for [99]. Here, auxin has been found to activate the mRNA specific for cellulase at very early periods, when there is no increased mRNA synthesis. The poly (A) – RNA isolated from treated segments, on translation in vitro, shows enhanced synthesis of cellulase. The poly(A) – RNA from control plants hardly codes for any cellulase. The above results suggest that the auxin perhaps activates the preexisting, dormant mRNAs into active translatable form of mRNA.
Thus, the evidences presented above indicate that most of the growth promoting hormones increases protein synthesis through increased mRNA synthesis as well as through activation of dormant mRNAs or the ribosomal system. Such dual action of phytohormones is well documented (165 and 166) ABA, in general, appears to exert an inhibitory effect on the synthetic processes. Although, ABA inhibits light-promoted unrolling and greening of etiolated leaves in wheat, it does not prevent light induced polysome formation [167]. In etiolated leaves of barley seedlings, ABA not only reduces the amount of polysome formation in the dark, but also inhibits light-induced increase in polysome size [168]. In barley aleurone cells, GA3 – induced a-amylase synthesis is inhibited by ABA and this inhibition is prevented by the addition of cordycepin. This observation indicates that ABA has both transcriptional as well as translational control [169]. In this context, increase in UMP-rich RNA component, in response to ABA treatment, is highly significant. Heywood et al. (116) have isolated a translational control RNA of mol.wt. 10,000 daltons, which is rich in UMP. This translational control RNA is believed to play a significant role in translational control where it inhibits translation of mRNAs, probably by pairing with poly-AMP segments of mRNAs [116 &170]. This observation correlates well with the effects of ABA on growth inhibition in plants.
Phytohormones and RNA-Protein Turnover;
The regulation of RNA and protein turnover is another important facet of cellular differentiation. Phytohormones are known to regulate such events. ABA, when applied to excised barley leaves, enhances chromatin bound nuclease and deoxy-ribonuclease (DNase) activities [180]. In excised Avena leaves, it affects one out of the four nuclease isoenzymes [171]. Similarly in lentils (Lens culnaris), ABA reduces the levels of RNA and also inhibits growth [172]. However, in maize coleoptiles, ABA suppresses the incorporation of radioactive precursors into RNA without affecting the levels of RNase for 8 hours [173].
Auxin is also implicated in suppressing the RNase activity in number of cases [174,175]. However, no direct relationship between nuclease levels and RNA stabilization has been established. In pea epicotyls, IAA elicits a large increase in membrane-bound RNase, which in turn is suppressed by kinetin [176]. Similarly, a correlation has been established between nucleases and auxin treatment in wheat root callus cultures [177].
In senescing leaf tissues, increased activities of RNase and DNase are correlated well with the decreased content of RNA and yellowing of the leaf. Cytokinins suppress such activities and delay senescence, which is popularly known as Richmond and Land effect [178-180]. In the absence of a “water stress” on leaves RNase activity is suppressed by kinetin but the same hormone under “water stress” condition enhances RNase activity [181].
In mustard and corn leaves, during senescence kinetin and 6-benzylaminopurine retard the loss of radioactive label from prelabelled proteins [182, 183]. In wheat leaves, cytokinin suppresses proteolysis of RuDP-carboxylase and this is prevented by CHI, suggesting that the proteolytic enzymes are being synthesized de novo during senescence [184].
During differentiation in Dictyostelium (from plasmodial stage to sporangium) [144], blue-green algae (vegetative to heterocyst) [185], and soybean callus cells (non-dividing to dividing state) [103], appearance and disappearance of some specific proteins have been noticed. This selective appearance and disappearance of certain proteins during developmental process may involve differential expression of genes. Such transitory proteins may play a very important role in morphogenesis.
Adventitious Root initiation as a morphogenetic system:
Introduction:
In the present investigation, hormone induced root initiation in the hypocotyls tissue of Phaseolus vulgaris Linn is used as a model system, to study the molecular events involved in the differentiation process. A brief review of the literature on adventitious root formation is therefore presented here.
The root that develops from any part of the plant body other than the radicle is called an adventitious root. Duhmel du Monceau (1758) for the first time explained adventitious root formation in stem as due to the downward movement of sap. Sachs (1880) postulated the existence of a highly potent root forming substance originating from leaves [186]. Van Tieghen and Douliot (1888) observed adventitious root formation in 67 dicotyledonous and 21 monocotyledonous families [187]. In 1933, Bouilleune and went propounded a hypothesis that “Rhizocauline”, a specific factor, is responsible for root initiation [188]. Went and Thimann (1937) observed for the first time that auxin, in addition to its growth-promoting effect, also induces root initiation in stem outings. Thus, a practical use was also found for the auxin [189].
Anatomical studies:
Previous anatomical studies reveal that during root initiation, meristematic activity is found in the region of endodermis, pericycle, especially over the vascular bundles [190-192]. However, there are many examples of adventitious roots originating from phloem parenchyma or phloem ray cells. It is also not uncommon to find roots arising from tissues such as primary xylem parenchyma, callus etc. [193]. The common observation of adventitious roots arising near vascular bundles, prompted Priestly and Swingle (1929) to suggest that nutrient supply may be a factor determining the site of root formation (194).
In recent years, pea epicotyls (Pisum sativum) and hypocotyls of Glycine max (soybean) have been used as systems for studying the effect of plant growth substances in the regulation of cambial activity and organization of root primordial in plants. Excised or intact hypocotyls, epicotyls segment and callus in response to auxin treatment, undergo dedifferentiation leading to the formation of root primordial. Preliminary anatomical studies have shown that parenchymatous cells, in the cortex of 24 hr auxin treated soybean hypocotyls and pea epicotyls, appear to be swollen and exhibit few cell divisions [72,195,196]. After 48 hr the number of cell divisions increase and disintegration of many cortical cells sets in, leaving lacunae. Root primordials originate from the cambial cells near vascular tissues and become recognizable after 3 days of hormone treatment. The primordial cells continue to divide and occupy the lacunae. In 4-5 days the new roots break through the epidermal layers of the segments.
Factors other than phytohormones:
Many environmental factors such as water supply, temperature, sunlight, acidity of the soil, etc. are known to affect root initiation. Young leaves promote root initiation but old leaves are found to inhibit them. Roots themselves inhibit new root formation, perhaps as a result of the production of some inhibitors [197].
The role of organic micronutrients is new root formation is significant. Adenine, arginine, aspargine and uracil promote root initiation [197] but guanine inhibits root formation in Phaseolus mungo. Among other organic micronutrients, nicotinamide, niacin, thiamin (vitamin B1), vitamin K an vitamin H and pyridoxyl enhance root formation only in the presence of auxin [197].
It is also interesting to note, that some cortisols stimulate adventitious root formation in the intact hypocotyls segments of mung beans [199]. The cortisol sensitive phase during root initiation is between 2nd and 4th day of treatment. However, cortisols in the presence of auxins, stimulate root initiation, but inhibit growth of the roots [200]. Recently it has been reported that vitamin D and its analogues by themselves promote root formation slightly, but in the presence of IBA, IAA or NAA, root production is greatly enhanced [201].
Genetics of root formation:
Zobel (1975) has described mutants in tomato with respect to root formation. These mutants are referred to as rosette (ro), lateral-les (dgt) and dwarf (drf), of which r has been established as adventitious rootless mutant [202]. The diploid chromosome number of these plants is 24 (2n = 24). Breeding experiments and genetic analysis have revealed that ro gene is located on the second chromosome and follows simple Mendelian inheritance. Hence, it is suggested that a single pair of alleles controls adventitious root formation.
Plant hormone interactions:
The most effective adventitious root forming substances known are IBA and NAA [188]. Staurt (1938) demonstrated that excised stem cuttings of Phaseolus mobilize carbohydrate reserves as soluble sugars within 24 hr of auxin treatment [203]. Altman and Wareing (1975) obtained similar results using [14 C]-CO2 in Phaseolus vulgaris cuttings [204]. However, the application of exogenous sugars could not replace the requirement of auxins for new root formation [205].
Ethylene stimulates root initiation in many leafy stem cuttings [206], but there are also cases where ethylene has no promotive effects [207,208]. Batten et al. (1978) have claimed that there is no correlation between the effectiveness in rooting and induction of ethylene production by a variety of auxins. Application of ethylene has no effect on root initiation and it proceeds normally even in the presence of 7% carbon dioxide or in hypobaric conditions [209]. In this context, it is pertinent to note, that in the hypocotyls of mung bean, IAA induces a specific protein, which in turn inhibits IAA induced ethylene production [210].
While ABA exhibits both stimulatory and inhibitory effects on new root formation [211,212], cytokinins and GA3 in general are inhibitory [213-215]. However, it has been suggested that the early stages of root formation are most sensitive to cytokinin and GA3 inhibition, but under certain conditions GA3 promotes root formation in Citrus embryoids [216]. Verga et al. (1974) have found that pretreatment of leaves with GA3, before excision, enhances adventitious root initiation. This effect is believed to be due to GA3 induced enhancement of auxin synthesis [217].
In callus cultures, in general, root formation is dependent on the ratio between auxin and cytokinin concentration. While higher ratios promote root formation, a lower ratio inhibits this process but induces shoot primordial. However, at intermediate ratios, both roots and shoots are produced. Hence, it is deduced that the relative concentration of auxin to cytokinin is very critical in the differentiation and development of organs such as roots and shoots [4] Fig.2.
Biochemical events:
During adventitious root initiation in the epicotyls of pea and hypocotyls of soybean, increase in DNA, RNA and protein contents has been observed. After 3 days of auxin treatment, cellulase activity increases 30-40 fold, pectinase 7-8 fold, pectin esterase and b 1 -> 3 Gluconase by 3-fold [195]. Similarly, during rhizogenesis, in detached cotyledons of mustard (Sinapis alba L.) increase in RNA and protein contents have been noticed [218]. Some evidence has been presented to show that the increase in cellulose activity is totally abolished, if the segments are treated with actinomycin D or azaguanine or puromycin along with the auxin. Hence, it is discerned that the continued synthesis of RNA and protein is essential for the increase in the activity of cellulase [196].
Trewavas (1976) has documented some interesting observations on the changes in nucleic acid metabolism and protein synthesis, specifically cellulose, during adventitious root initiation in pea epicotyls and soybean hypocotyls [196] (Fig. 3) In epicotyls of pea plant, over a 3 day period, auxin causes an increase in DNA and protein contents by 2.5 fold and RNA content 4.0 fold. In soybean hypocotyls, a 4-5 fold increase in DNA and protein contents and a 10-foldincrease in RNA content have been observed. Under similar conditions, actinomycin D and puromycin inhibit the auxin induced increase in DNA, RNA and protein contents.
In the presence of auxin, the ratio of microsomal RNA to soluble RNA is 6.9 compared to the control value of 4.8. These changes are attributed to auxin induced differential turnover of RNA. Furthermore, labeling studies have indicated that initial lag period for auxin mediated increase in RAN synthesis is l hr in pea segments and 3 Hr in soybean. After several hours of treatment with auxin, increased labeling has been found in rRNA and tRNA [72,151].
Using in vitro transcriptional assay system and DNA-RNA hybridization techniques, O’ Brien et al. (1968) and Verma et al. (1975) have demonstrated qualitative and quantitative changes in RNA [79,83]. This implies selective unmasking of the genome, which correlates well with the time point at which cell divisions start.
In a time-course study in pea segments, Davies et al (1963) have detected a further shift in the labeling pattern of the polysomal populations. At the end of the third hour the proportion of polysomes containing 10 ribosomes or more has increased 3 fold. Ribosomal populations double within 6-10hours. After 10 hr of treatment, the ratio of polysomes to monosomes reaches a value of 9, compared to 0.5 in control tissue [219].
In soybean hypocotyls a shift from monosomes to polysomes is noted after 3 hr of auxin treatment and this is sensitive to actinomycin D, but not to 5-FU, 6-methylpurine in polysome size is independent of ribosomal RNA synthesis, but dependent upon the continued synthesis of tightly bound (TB) and DNA-like RNA (D-FNA). It has been further demonstrated that the increase in protein synthesis by individual polysomes in vitro is due to the increased activation of chain initiation, probably through the synthesis of 3 specific 60s ribosomal proteins [77,166].
The present investigation has been undertaken to study (1) the early molecular events leading to dedifferentiation of pericyclic cells into root primordial; (2) the identification of specific gene products essential for adventitious root initiation; (3) a delineation of sequence of events at molecular level leading to the emergence of root initials. Adventitious root initiation in hypocotyls segments of Phaseolus vulgaris Linn. In response to the hormone IBA treatment has been used as the model system.
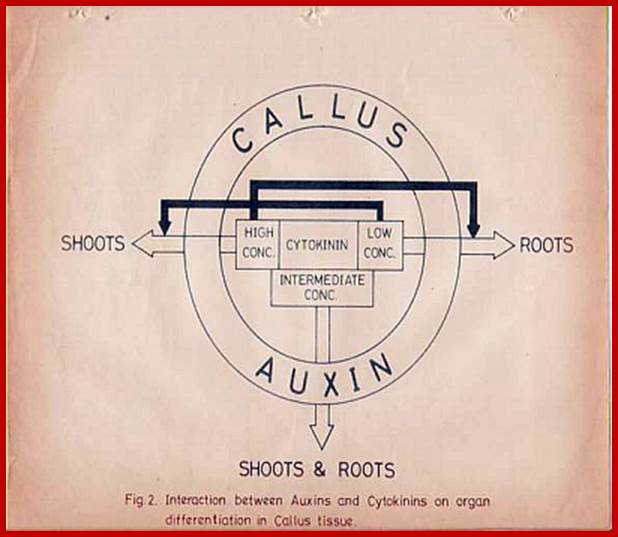
GrkRaj
Interaction between Auxins and Cytokinins during Organ differentiation in callus cultures. Open arrows indicate the promotion of organs and closed arrows indicate inhibition.
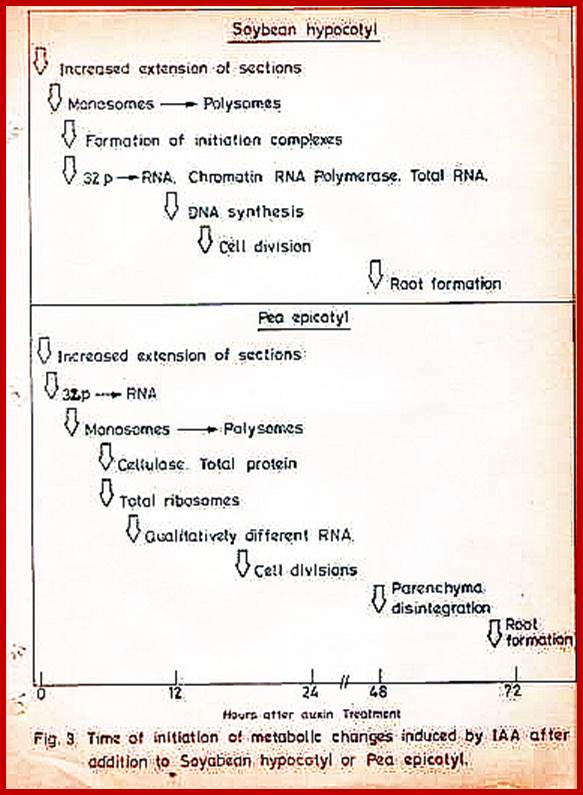
Grkraj; PhD thesis 1979
Time of initiation of metabolic changes induced by IAA after addition to Soybean hypocotyls or pea epicotyls.
Phytohormones and immunity:
Plant growth and response to environmental cues are largely governed by phytohormones. The plant hormones ethylene, jasmonic acid, and salicylic acid (SA) play a central role in the regulation of plant immune responses. In addition, other plant hormones, such as auxins, abscisic acid (ABA), cytokinins, gibberellins, and brassinosteroids, that have been thoroughly described to regulate plant development and growth, have recently emerged as key regulators of plant immunity. Plant hormones interact in complex networks to balance the response to developmental and environmental cues and thus limiting defense-associated fitness costs. The molecular mechanisms that govern these hormonal networks are largely unknown. Moreover, hormone signaling pathways are targeted by pathogens to disturb and evade plant defense responses. In this review, we address novel insights on the regulatory roles of the ABA, SA, and auxin in plant resistance to pathogens and we describe the complex interactions among their signal transduction pathways. The strategies developed by pathogens to evade hormone-mediated defensive responses are also described. Based on these data we discuss how hormone signaling could be manipulated to improve the resistance of crops to pathogens. Nicolas Denancé, et al; http://journal.frontiersin.org/
Hormone Treatments Trigger Widespread Effects on Hormone Metabolism;
Network of Hormone Effects on Hormone Metabolism
Genes assigned to hormone biosynthetic pathways by GO annotation were identified within lists of hormone-responsive genes. Lines with arrowheads represent upregulation of hormone biosynthetic genes or downregulation of genes involved in hormone inactivation. Blocked arrows represent downregulation of genes involved in hormone biosynthesis or upregulation of genes involved in inactivation of a hormone. Diamond arrowheads indicate changes in gene expression with ambiguous outcomes (i.e., genes affected include those linked to both increased and decreased hormone levels).
Despite the low numbers of shared transcriptional targets, there was ample evidence of one hormone regulating genes involved in the metabolism of another hormone. This phenomenon has been suggested for many hormones and is particularly well-documented for the production of ethylene following IAA treatment (Alonso and Ecker, 2001). ACC synthase (ACS) catalyzes the rate-limiting step of ethylene biosynthesis, and several hormones have been shown to affect the levels of ACS genes ( Wang et al., 2002). Here, ACS6 (At4g11280) was upregulated by ABA, BL, and IAA. ACS10 (At1g62960) was downregulated by ABA, IAA, MJ, and GA. The complex web of such interactions ( Figure 4) suggests that long-term effects of all hormone treatments represent a “domino effect,” resetting many systems within the plant.
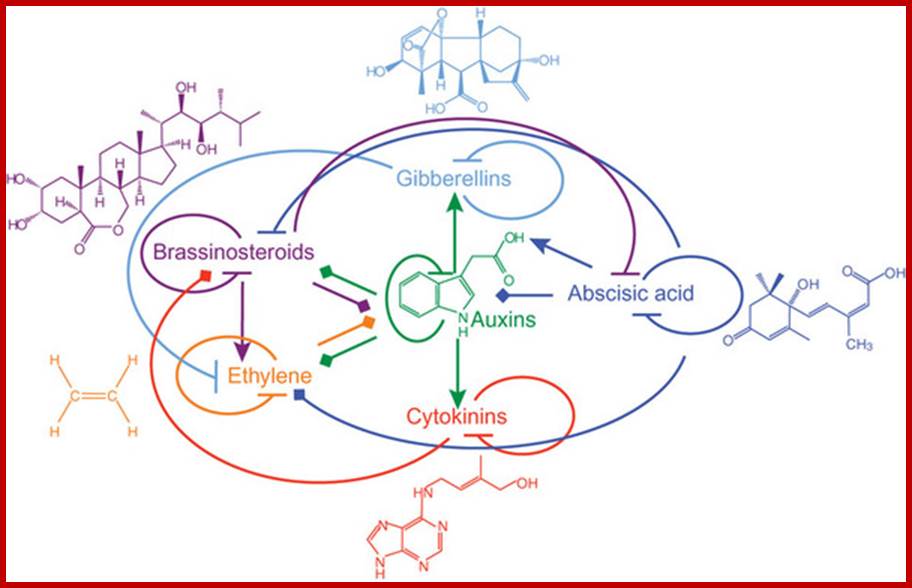
Plant hormones play a major role in plant growth and development. They affect similar processes but, paradoxically, their signaling pathways act nonredundantly. Hormone signals are integrated at the gene-network level rather than by cross-talk during signal transduction. In contrast to hormone-hormone integration, recent data suggest that light and plant hormone pathways share common signaling components, which allows photoreceptors to influence the growth program. We propose a role for the plant hormone auxin as an integrator of the activities of multiple plant hormones to control plant growth in response to the environment. http://www.nature.com/