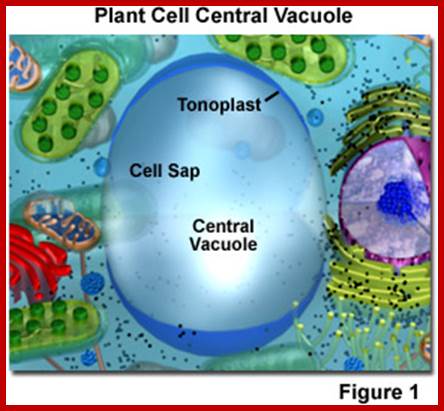
Plant Cell Vacuoles
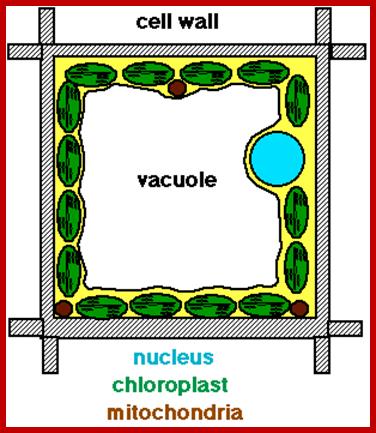
Mature plant cells contain a single large central vacuole and it is the most conspicuous compartment of the cell which occupies nearly 50-70% of the total cell volume. On the contrary, meristematic cells are lacking in such vacuoles. Plant cell vacuoles are distinct and characteristic in having a single unit membrane called tonoplast, which separates the vacuolar content from the rest of the cytoplasmic fluid. The liquid present within the vacuole is often called as cell sap which contains a host of inorganic and organic compounds.
Cell derivatives of meristems; first undergo expansion, and then differentiation. During this stage many smaller vacuoles arise from cytoplasmic membranes of both RER and SER and fuse with one another to form a large central vacuole.
Tonoplast membranes at their cytoplasmic surfaces are associated with polysomal complexes engaged in protein synthesis. In the course of time the central vacuole gets loaded with wide variety chemical components. The most perplexing situation is that during transformation of mature parenchyma cells into meristematic cells the central vacuole disappears. What happens to the vacuolar components is not known.
Plant vacuoles can be identified from other membranous vesicles by vital staining techniques. The neutral red stain being basic in its properties binds components of cell vacuole. These are highly inducible enzymes. ER is involved in the intracellular transportation of various components like proteins, lipids, carbohydrates and others via golgi complex. Some of ER membranes, containing specific proteins within their lumen, are pinched off into vesicles which later fuse with each other or get processed via Golgi complex and get integrated with specific cell organelles. Even cellular vacuoles of various kinds and dimensions show metabolic activities like amino acid metabolism, fatty acid oxidation etc.
The loading of variety of components in to cell vacuoles bring about changes in the osmotic potential of the cell sap. As a consequence of this, water may freely enter into protoplast; this has been taken as evidence to argue that tugour changes within the cell sap is mainly responsible for the growth. But now it is known that the growth of the cell takes place without development of turgour pressure.
Vacuolar expansion and concentration is another dynamic feature, where vacuoles play a predominated role in the development of young buds. The swelling of vacuole forces the cell wall to bulge into a bud. Similarly in the case of stomata, guard cells contain a number of small contracted vacuoles, but at the time of opening, the same vacuoles fuse and enlarge into a large central vacuole in the guard cells, thus they bring about the movement of guard cells. Nonetheless, it is speculated that hormones like Abscisic acid and Cytokinin play important roles in closing and opening of stomata
Vacuolar Contents:
Inorganic substances found in the vacuole show variation from cell type to cell type. For example, more than 90% of the total cellular Mg2+ ions are found within the vacuole. On the contrary, the total concentration of calcium ions and copper ions is just 6% and 2% respectively. But the most common ions like K+ ions are equally distributed between cytoplasm and vacuoles. In certain cases more than 40% of the total phosphates are found in vacuoles and most of it is in the form of polyphosphates called
Volutin threads.
http://micro.magnet.fsu.edu/
Enzymatic components:
It is really interesting to observe the presence of a wide variety of hydrolysing enzymes within the vacuoles. The common enzymes found are carboxypeptidase, RNase, DNase, phosphotases, b-glycosidase, alfa and beta–amylase etc. The concentration and composition of such enzymes vary from cell to cell and species to species, strangely leaf cells of Solanaceae members like tomato, potato, contain significant amount of protease inhibitors within their vacuoles. How such variety of enzymes and other components are retained and released is a mystery.
Other organic compounds:
Plant cell vacuoles also contain a good number of organic carboxylic acids, amino acids, amides, mucilage, anthocyanins, flavones, gums, alkaloids, anthocyanin and other pigments and even tannins. In certain plants like citrus, the vacuoles are filled with highly acidic citrate compounds whose pH is about 2.5 and curiously enough such acidic pH is prevented from inactivating cytosolic components by tonoplast membranes and provides a distinct compartmentalization. In some cases more than 25 per of the total amino acid pool is found in vacuoles.
Specialized Vacuoles:
Aleurone vacuoles: Certain vacuoles in seeds or grains of various pulses and cereals store a variety of proteins; such granular components are referred as aleurone grains. Inulin, legume, vanillin, glycerin, etc. are some of the common proteins found as storage products in Aleurone vesicles. Such vesicles are derived from RER-SER membrane transitions. Most of the stored or aggregated nonliving structures are called ergastic substances.
Spherosomes and starch vacuoles: Similar to Aleurone vesicles, lipids and starch are also stored in special vacuoles called spherosomes and amylosomes. Spherosomes are derived from RER-SER transitional endomembrane. Such membranous vesicles are endowed with enzymatic system from synthesis and inter-conversion. On the other hand, starch is synthesized in chloroplasts but transported to be stored in amyloplasts. Such starch containing membranous organs are found in large numbers in storage tissues like root tubers, stem tubers, cotyledons and endosperms of seeds and grains. The role of pyrenoids found in lower algal cells and their role in storing starch is very interesting.
Functions:
Vacuoles are highly dynamic. They store many inorganic and organic compounds including a host of enzymes. At the same time they are engaged in protein synthesis at the cytosolic surfaces of tonoplast where they of found as polysomal complex. Some of the ions are transported across tonoplast membranes against concentration gradient. In crassulacian plants carboxylic acids are released into cytoplasm at nights. The diurnal behavior of vacuoles is a unique feature for succulents. The synthesis and aggregation of various substances like calcium oxalate crystals, raphides and other ergastic is very common for plant central vacuoles.
http://xarquon.jcu.cz/edu/
Isolated central vacuole; http://www.biology.arizona.edu
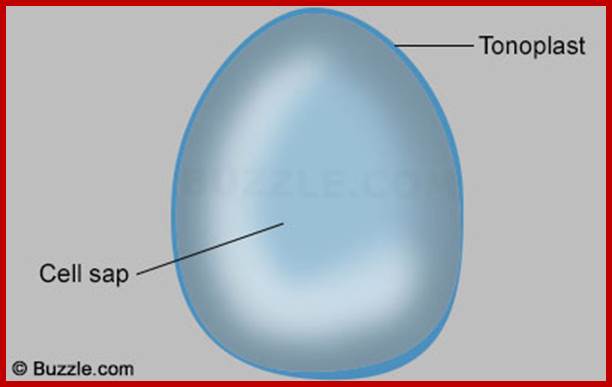
Organelles and their functions; http://www.buzzle.com/

Tonoplast with peripheral protoplasm; http://xarquon.jcu.cz/edu/
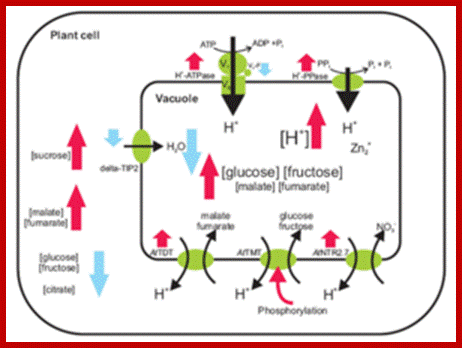
https://systembiologie.uni-hohenheim.de
Tonoplast membrane proteins play a very important role protein phosphorylation and protein targeting.

Membrane transport system in plant cells; www.http://www.cosmobio.co.jp/
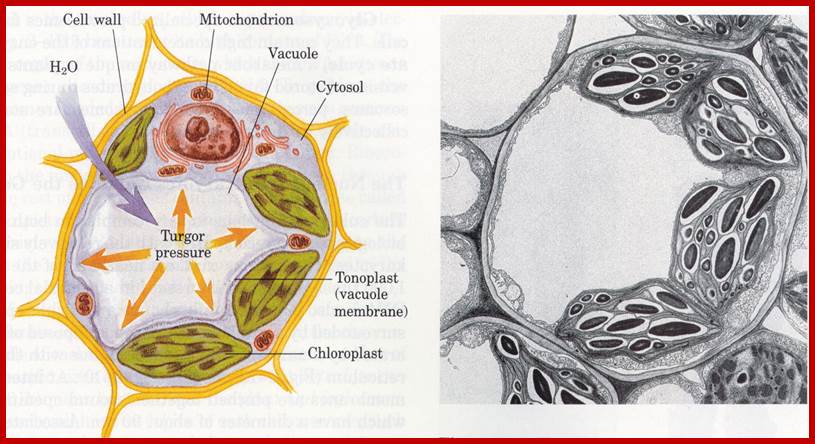
Tonoplast membrane is involved in transportation many components across both inwards and out words. It is involved in developing turgidity of the cells. They are lytic compartments, function as reservoirs for ions and metabolites, including pigments, and are crucial to processes of detoxification and general cell homeostasis. They are involved in cellular responses to environmental and biotic factors that provoke stress.
Plant Cell central Vacuoles;
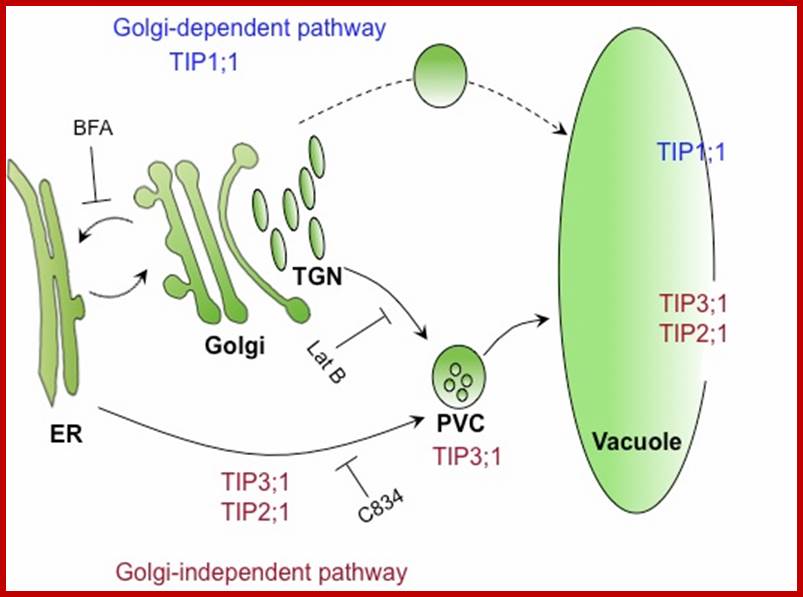
They act like animal lysosomes. They can breakdown large molecules under acidic conditions for the vacuole sap is acidic. Plant central vacuole develops from smaller provacuolar structures and Golgi vesicles. As plant cell expands the central vacuole increase in size and occupies more than 90% of the cell space and the all cell organelles pushed to peripheral region. Central vacuole is formed due to of multiple membrane vesicles. In Arabidopsis, TIP3;1 and TIP2;1 are likely to use a Golgi-independent pathway, while TIP1;1 is likely to use a Golgi-dependent pathway. Tonoplast has a regulatory role in movement of ions. The Vacuole acts as storage organ, also store toxic components, it provides pH and ionic homeostasis; it also acts a defense organelle.
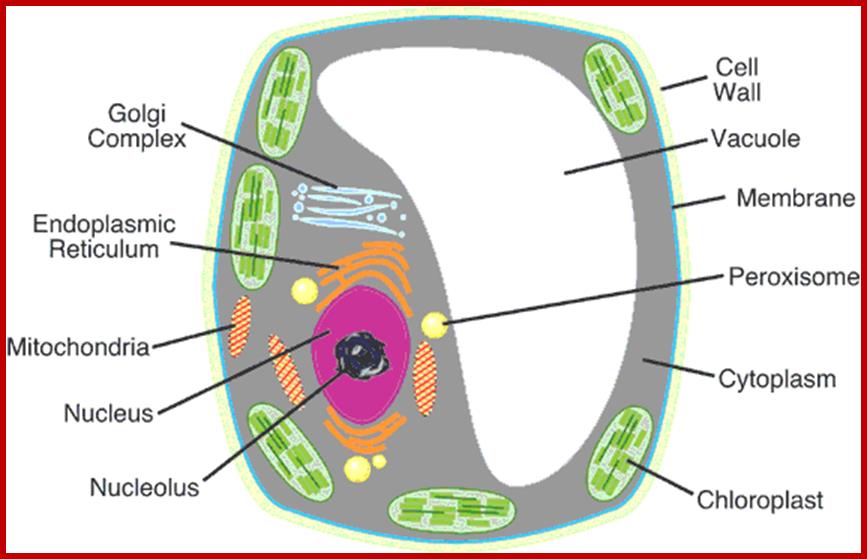
http://www.plantcell.us/
The central vacuole is bound by a membrane called Tonoplast, which contains many transporters. It contains several enzymes such as acid hydrolases, proteases, ribonucleases and glycosidases. In plant leaves stomatal opening (K ions in ) and closing (k ions out) is performed by many ions inflow and out flow of some across central vacuole and plant cell membrane. Important ion flow is K ions coupled to Hydrogen ions facilitated by ATP pumps.
When conditions are conducive to stomatal opening (e.g., high light intensity and high humidity), a proton pump drives protons (H+) from the guard cells. This means that the cells' electrical potential becomes increasingly negative. The negative potential opens potassium voltage-gated channels and so an uptake of potassium ions (K+) occurs. To maintain this internal negative voltage so that entry of potassium ions does not stop, negative ions balance the influx of potassium. In some cases, chloride ions enter, while in other plants the organic ion malate is produced in guard cells. This increase in solute concentration lowers the water potential inside the cell, which results in the diffusion of water into the cell through osmosis. This increases the cell's volume and turgor pressure. Then, because of rings of cellulose microfibrils that prevent the width of the guard cells from swelling, and thus only allow the extra turgor pressure to elongate the guard cells, whose ends are held firmly in place by surrounding epidermal cells, the two guard cells lengthen by bowing apart from one another, creating an open pore through which gas can move.
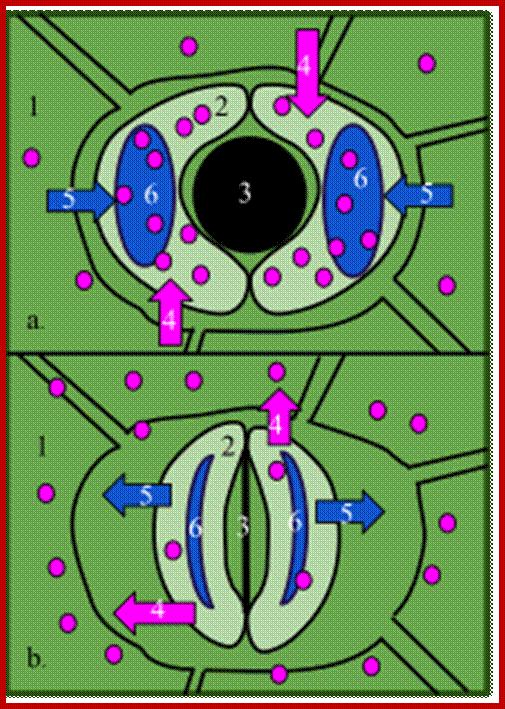
When stomata are closed (at the end of the dark period), the vacuolar membrane potential is close to 0 mV and cytosolic malate concentrations are low (left panel). In these conditions, AtALMT9 is not active and does mediate anion accumulation into the vacuole. During stomatal opening (right panel), the membrane potential of the vacuole drops to values close to −60 mV. Starch is degraded to malate, which is also taken up from the apoplast inducing a raise of the cytosolic malate concentration. These conditions activate AtALMT9. At the same time, chloride enters into guard cells. To sustain the opening of stomata, solutes have to be accumulated in the vacuole of guard cells to increase the water potential. In this phase, AtALMT9 mediates chloride accumulation in the vacuole.
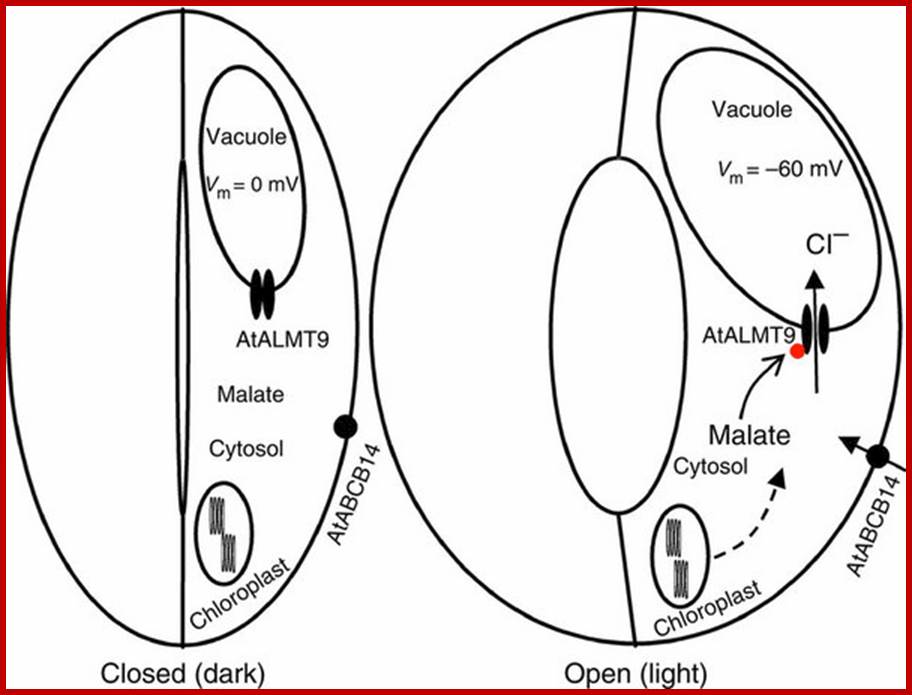
Model for the role of AtLMT9 in stomatal opening; http://www.nature.com/
When the roots begin to sense a water shortage in the soil, abscisic acid(ABA) is released. ABA binds to receptor proteins in the guard cells' plasma membrane and cytosol, which first raises the pH of the cytosol of the cells and cause the concentration of free Ca2+ to increase in the cytosol due to influx from outside the cell and release of Ca2+ from internal stores such as the endoplasmic reticulum and vacuoles. This causes the chloride (Cl−) and inorganic ions to exit the cells. Second, this stops the uptake of any further K+ into the cells and, subsequently, the loss of K+. The loss of these solutes causes an increase in water potential, which results in the diffusion of water back out of the cell by osmosis. This makes the cell plasmolysed, which results in the closing of the stomatal pores.
Guard cells have more chloroplasts than the other epidermal cells from which guard cells are derived. Their function is controversial; Wikipedia. Here we present the molecular identification of the long-sought-after vacuolar chloride channel. AtALMT9 is a chloride channel activated by physiological concentrations of cytosolic malate. Single-channel measurements demonstrate that this activation is due to a malate-dependent increase in the channel open probability. Arabidopsis thaliana atalmt9 knockout mutants exhibited impaired stomatal opening and wilt more slowly than the wild type. Our findings show that AtALMT9 is a vacuolar chloride channel having a major role in controlling stomata aperture “www.nature.com”
https://www.cals.ncsu.edu
www.slideplayer.com
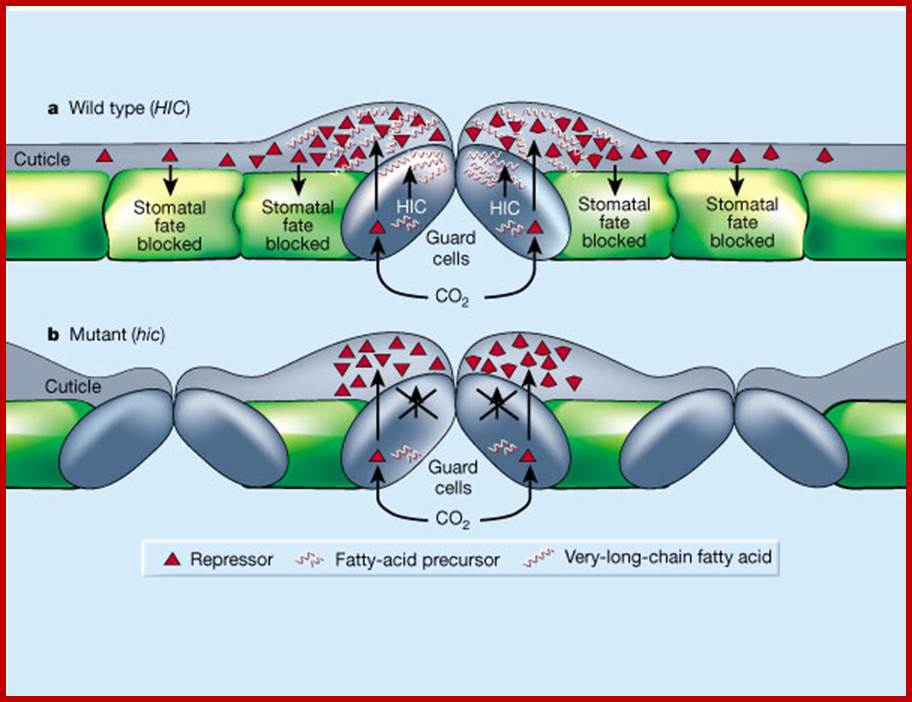
Over the past 200 years, the number of plant stomata (each of which consists of a pair of guard cells, delimiting a pore for gas exchange) has decreased in response to increasing CO2 levels2, 3. This trend may not continue in the future. Gray et al.1 show that the stomatal index remains the same in normal Arabidopsis plants in response to CO2 concentrations double those of today, but increases in plants with a mutant HIC gene. The authors suggest that the normal HIC gene negatively regulates stomatal development at the higher CO 2 concentrations, as shown here. a, The normal HIC gene may encode an enzyme involved in the synthesis of very-long-chain fatty acids, deposited at the surface of the guard cells. These fatty acids might be needed for the diffusion of a molecule — stimulated by high CO2 concentrations — that can reach neighboring epidermal cells and repress their ability to develop into stomata. b, In hic mutant plants, the relevant fatty acids are not made. Diffusion of the repressor is therefore impaired, so it cannot reach all its target cells, which develop into stomata; Nature .com
http://www.nature.com/
Functions of the vacuoles include:
· Isolating materials that might be harmful or a threat to the cell
· Containing waste products
· Containing water in plant cells
· Maintaining internal hydrostatic pressure or turgor within the cell
· Maintaining an acidic internal pH
· Containing small molecules
· Exporting unwanted substances from the cell
· Allows plants to support structures such as leaves and flowers due to the pressure of the central vacuole
- In seeds, stored proteins needed for germination are kept in 'protein bodies', which are modified vacuoles.
- Vacuoles also involved in autophagy.
- They also aid in the lysis and recycling of misfolded proteins that have begun to build up within the cell.
- They also aid in the lysis and recycling of misfolded proteins that have begun to build up within the cell; www.en.wikipedia.org.