Protoplasmic Cytoskeleton Elements
The protoplasmic liquid without any cell organelles is known as hyaloplasm. This part of the protoplasm is known to perform various metabolic activities like glycolysis, amino acid metabolism, EMP pathway, fatty acid oxidation, charging of tRNA, protein synthesis and many others. These are executed by soluble enzymes.
It was believed in the past that such hyoloplasmic fluid is free from any other structures and it is just a clear liquid part containing host of enzymes. But electron microscopic studies, made it clear that the fluid portion contains a large number of fine filamentous structures of various sizes and dimensions. Such structures were once believed to be the artifacts of methods employed for electron microsocpy. Now the complex network of filaments has been identified as cytoskeleton fabric. In fact, the entire cytoplasm is pervaded with cytoskeleton material and various functions have been attributed to them such as mechanical support to cytoplasm, determination of cellular shapes, intracellular transportation, protoplasmic streaming, cell polarity fixation, cytoplasmic cleavage, protein synthesis, etc. Protoplasm is a dynamic fluid.
Protoplasm also contains what is called ergastic substances; they are nonliving components like crystals, tannins, resins, oil globules starch granules etc. Protoplasm also contains different active organelles like mitochondria, golgi bodies, lysosomes, plastids, vacuoles and the most important organelle ‘The Nucleus’.
Isolation of cytoskeletal components by Sol-Gel transformations and differential high- speed centrifugation techniques has been made it easy. Isolation and purification of them greatly helped in understanding the chemical composition, 3D structure and functions of these components. The network of these filaments is so extensive they are virtually associated with all kinds of organelles and membranes, with the exception mitochondria. But mitochondria are associated with ER and ER is associated with cytoskeletons. (Contrasting explanation in different texts). The cytoskeletal fabric is primarily made up of four to five components like microtubules, microfilaments, and microtrabaculae and intermediary filaments. They are associated with each other as structural and functional components. Sometimes the functions performed by them overlap because their functions are coordinated actions.
The intracellular fluid-cytosol is a crowded solution of many different types of molecules and structures that fills much of the volume of cells. http://en.wikipedia.org/
Microtubules:
Microtubules are tubular protein structures with a diameter of 250A-280A and several micrometers in length. The wall of such tubule is 50A thick. A large number of such tubules from a network and they are associated with various membrane structures like plasma membrane, ER, etc. They form an important component of centrioles (animal cell), kinetochores and mitotic apparatus. It is also surprising to find that more than 20% of the total proteins of brain cells are microtubules. Plant cells are no exception to the presence of such fibers and in actuality they are found distributed all over in the cytoplasm. Further more cilia, flagella, basal granules found in unicellular, motile algae are made up of microtubules. (Refer to proteins).
Cytoskeletons; Wikipedia
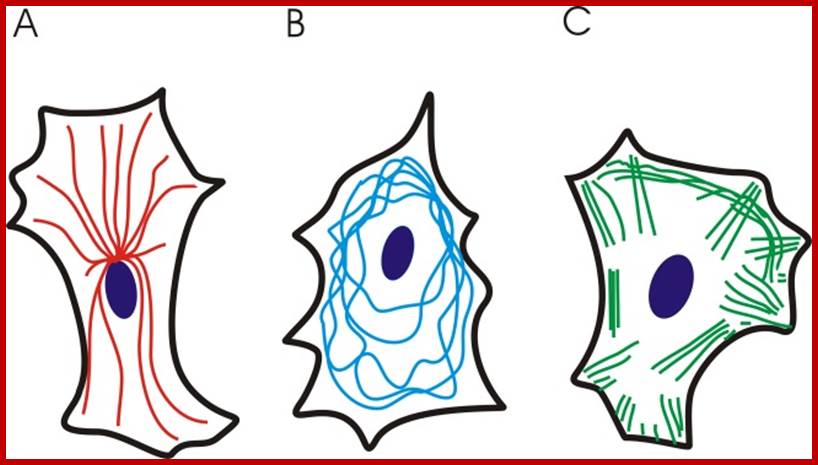
Schematics for three different distribution patterns of cytoskeletal filaments: (A) Microtubules (red) are hollow cylindrical filaments with a diameter of 25 nm, radiating from the cell nucleus. Microtubules are organized by the microtubule organizing centers (centrioles and basal bodies). They are capable of growing and shrinking to generate force. They can be used by motor proteins that support the movement of organelles and other cellular factors along the microtubules. (B) The intermediate filaments (blue) have an average diameter of 10 nm, and they are mostly cytoplasmic (except the nuclear filaments known as lamins). These filaments are deformable proteins that can be stretched to several times their initial length. Such a large deformation is possible due to their hierarchical structure (Qin et al., 2009). (C) The actin microfilaments (green) are the thinnest cytoskeletal filaments (7 nm in diameter). They are assembled in highly versatile bundles and networks, they can be cross-linked and they resist both tensile and compressive forces. They are distributed both in the cortical cytoplasm and throughout the entire cell. They are able to generate force by either elongating or depolymerizing, and they work as part of the actinoomyosin molecular motor (Alberts et al., 2002). http://www.intechopen.com/
Chemical Composition and Structure:
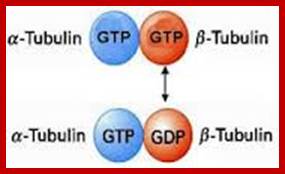
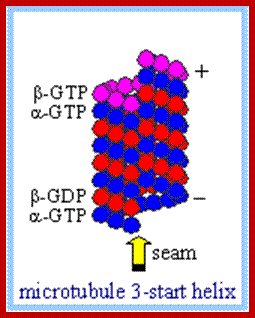
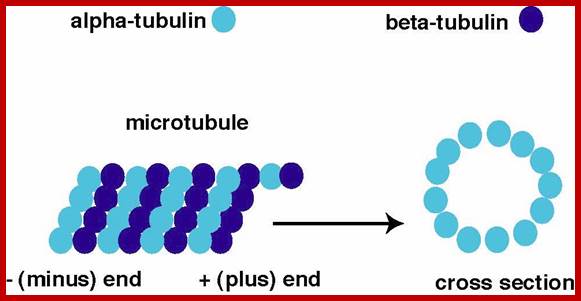
Microtubules are made-up of protein subunits like α & β tubulins of mol. wt 55-58 KD and the heterodimer is 100kDa. They are coded for by many genes- multigene family. In humans there are 15 to 20 gene sequences for each of alpha and beta type Tubulins. Once translated they are modified such as phosphorylation, tyrosination, acetylation or and glutamylation, this can be tissue type. Alpha and beta subunits have conserved amino acid sequences by 72-78%, it is only carboxy terminal shows some variations. They are also associated with another class of proteins called microtubule associated proteins or MAPs of mol. wt. ranging from 55-62 kDa, some are 200-kDa or more (four classes like MAP1 to MAP-4, they are called Tau proteins-T. Alpha tubulins have different types called TUB1A, TUBB, TUBC, TUBD, TUBE and thy classified into different classes. Even beta tubulins have different classes such as class III Beta, beta1 and VI beta and sub types. Most of the drugs bind to beta tubulins. There are Y-Tubulins, TUB G, and delta and epsilon Tubulins. With respect to amino acid composition α & β tubulins show greater similarity with one or two different amino acids residues. Tubulins isolated from different species of plants and animal are remarkably same in amino acid composition, sequence and structure. Thus, tubulins as the most conserved molecules in the biological world. These subunits are globular in shape and posses binding sites for GTP. They first polymerize into dimers and then they grow into the length of 50µm tubular filament, its outer diameter is 24nm and inner diameter is 12nm. Tubulins do undergo modifications at C-terminal region (rich in glutamate) of alpha –tubulin. Half-life of microtubules is 5-10 minutes, but some stay stable for hours. Colchicine or vinblastine by binding to tubulin units prevents polymerization of them into microtubular filaments. Calcium also inhibits polymerization. Homologs of α and β tubulins, called BtubA and BtubB are also found bacteria called Prostheobacter. They form microtubules in vivo called protofilaments. TubZ bacteria and Archaea function in cell division in segregating plasmids
Microtubules
are polarized tubular structures that are produced by the linear polymerization
of ![]() - and
- and ![]() -tubulin heterodimers into protofilaments.
These assemble to form a hollow fiber of 13 tubulins. The addition of tubulin
subunits preferentially occurs at the plus end. Microtubule assembly is
intrinsically dynamic and consumes energy. Incorporation into microtubules
requires a GTP-bound form of
-tubulin heterodimers into protofilaments.
These assemble to form a hollow fiber of 13 tubulins. The addition of tubulin
subunits preferentially occurs at the plus end. Microtubule assembly is
intrinsically dynamic and consumes energy. Incorporation into microtubules
requires a GTP-bound form of ![]() -tubulin. Within the
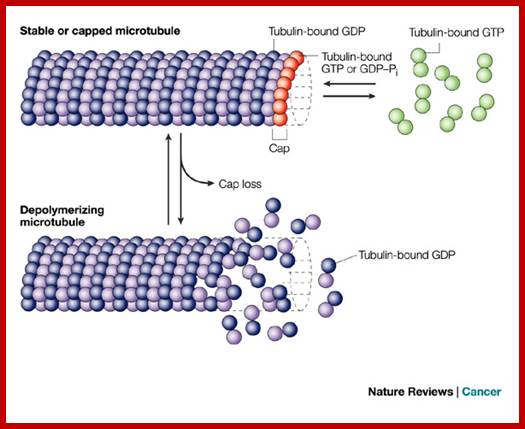
microtubule lattice, GTP is hydrolyzed to GDP. In many cell types, the minus
end of microtubules is embedded in the Microtubule
organizing center (MTOC), which is
tightly linked to the centrosome. The MTOC prevents shrinkage from the
microtubule minus end, so dynamic instability occurs at microtubule plus ends.
Under such conditions, microtubules alternate between phases of growth, pause
and shrinkage at their plus ends. Conversion from growth to shrinkage is termed
'catastrophe', whereas the switch from shrinkage to growth is called 'rescue'.
If the minus end is not embedded, dissociation of GDP-bound tubulin subunits
can occur at these ends. After conversion to GTP-bound tubulin, the subunits
can be incorporated at the plus end, so that tubulin subunits 'treadmill'
through the microtubule (see figure; red indicates GTP-bound
-tubulin. Within the
microtubule lattice, GTP is hydrolyzed to GDP. In many cell types, the minus
end of microtubules is embedded in the Microtubule
organizing center (MTOC), which is
tightly linked to the centrosome. The MTOC prevents shrinkage from the
microtubule minus end, so dynamic instability occurs at microtubule plus ends.
Under such conditions, microtubules alternate between phases of growth, pause
and shrinkage at their plus ends. Conversion from growth to shrinkage is termed
'catastrophe', whereas the switch from shrinkage to growth is called 'rescue'.
If the minus end is not embedded, dissociation of GDP-bound tubulin subunits
can occur at these ends. After conversion to GTP-bound tubulin, the subunits
can be incorporated at the plus end, so that tubulin subunits 'treadmill'
through the microtubule (see figure; red indicates GTP-bound ![]() -tubulin subunits, whereas
green indicates GDP-bound
-tubulin subunits, whereas
green indicates GDP-bound ![]() -tubulin subunits). To
visualize 'tread milling' in the figure, specific microtubule subunits are
marked with an asterisk and the microtubule lattice is marked with a grey
rectangle.
-tubulin subunits). To
visualize 'tread milling' in the figure, specific microtubule subunits are
marked with an asterisk and the microtubule lattice is marked with a grey
rectangle.
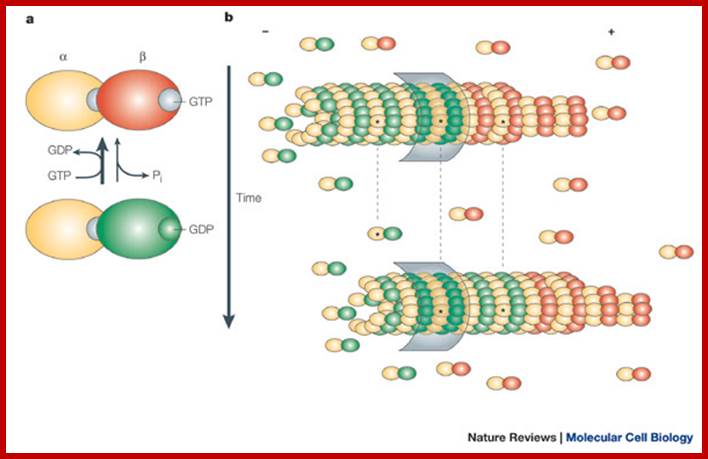
When
microtubules are viewed using cryo-electron microscopy in vitro,
growing ends appear as a curved sheet of protofilaments, whereas shrinking ends
contain bent or 'peeling' protofilaments that are known as 'rams horns'. A
GTP–tubulin cap is thought to stabilize microtubule plus ends, whereas a
GDP–tubulin cap induces catastrophe, presumably because GDP–tubulin
protofilaments assume a curved conformation. In vivo, stable, long-lived microtubules might therefore
have a cap of GTP–![]() -tubulin and/or a sheet of
protofilaments, or are otherwise protected from destabilizing effects. Stable
microtubules acquire several post-translational modifications, such as
acetylation of
-tubulin and/or a sheet of
protofilaments, or are otherwise protected from destabilizing effects. Stable
microtubules acquire several post-translational modifications, such as
acetylation of ![]() -tubulin, or proteolytic removal
(detyrosination) of the C-terminal tyrosine of this subunit; http://www.nature.com/
-tubulin, or proteolytic removal
(detyrosination) of the C-terminal tyrosine of this subunit; http://www.nature.com/

Alpha and beta subunits for heterodimers alpha binds to GTP and but don’t hydrolyze, but beta binds to GTP or GDP but it can hydrolyze GTP to GDP and Pi and it can exchange GDP for GTP; http://www.rpi.edu/
http://www.angelfire.com/
http://www.rpi.edu/
Several studies revealed that beta tubulin was decreased in the anterior cingulate cortex (ACC) of patients with schizophrenia. http://thepsychguru.com/
To form microtubules, the dimers of α- and β-tubulin bind to GTP and assemble onto the (+) ends of microtubules while in the GTP-bound state. The β-tubulin subunit is exposed on the plus end of the microtubule while the α-tubulin subunit is exposed on the minus end. After the dimer is incorporated into the microtubule, the molecule of GTP bound to the β-tubulin subunit eventually hydrolyzes into GDP through inter-dimer contacts along the microtubule protofilament. Whether the β-tubulin member of the tubulin dimer is bound to GTP or GDP influences the stability of the dimer in the microtubule. Dimers bound to GTP tend to assemble into microtubules, while dimers bound to GDP tend to fall apart; thus, this GTP cycle is essential for the dynamic instability of the microtubule. The Α α -tubulin has eight subtypes and β- tubulin has nine subtypes; besides the main types there are gamma, delta and epsilon types of subunits.
Highly dynamic mitotic-spindle microtubules are among the most successful targets for anticancer therapy. http://www.nature.com/

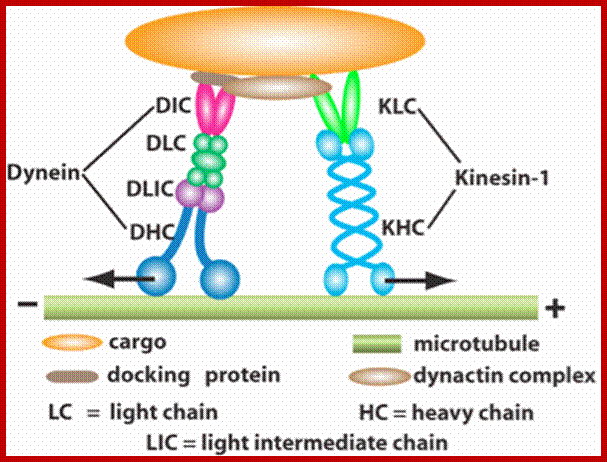
Components of the microtubule-based axonal transport system; a | Kinesin and dynein complexes govern the axonal transport of most cargoes. Kinesin, which is composed of two heavy chains and two light chains, moves cargoes in the anterograde direction along axons (that is, towards the axon tip and the plus end of microtubules). Dynein complexes, which comprise the dynein heavy, intermediate, intermediate light and light chains and several Dynactin subunits (such as DCTN1 and DCTN2), move cargoes in the retrograde direction (that is, towards the minus end of the microtubules). Both Kinesin and dynein motor proteins have a glomerular motor domain in their heavy subunits that binds to microtubules and hydrolyses ATP to propel cargoes along the microtubule rails. b | Cargoes (including mitochondria and vesicles) are attached to kinesin by the motor protein's light subunit. Dynactin is a complex involved in the attachment of the cargo onto dynein. Myosin Va is involved in the transport of cargoes along actin filaments. It has two head motor domains that bind to actin microfilaments and ATP, two α-helical segments that bind to Calmodulin and two glomerular tail domains that bind cargoes. Myosin Va cooperates with the microtubule motors to regulate the distribution of cargoes along the cytoskeleton; www.Nature.com
The assembly of tubulin subunits requires nucleating centers which are normally found associated with plasma lemma, kinetochores and centrosome. Presence of GTP as energy supply and Mg2+ ions, α and β tubulin subunits assemble on nucleating structures and grow into long tubular proteins. They have (-) end and the (+) end, where tubulin subunits are removed and tubulin subunits are added respectively. The assembly of tubulin monomers also requires Map proteins. In fact, Maps play a very important role not only the assembly, but also they are believed to control the orientation and alignment of micro tubules within the cells. There always exists a dynamic equilibrium between the microtubules and tubulin monomeric pool in the cytoplasm. Microtubules are highly sensitive to cold temperature at which they undergo deploymerization rapidly into monomers; but they immediately assemble into tubular proteins when the temperature is restored. The assembly and disassembly is very rapid and very well regulated. In living cells, microtubules are also associated with microfilaments and microtrabaculae.
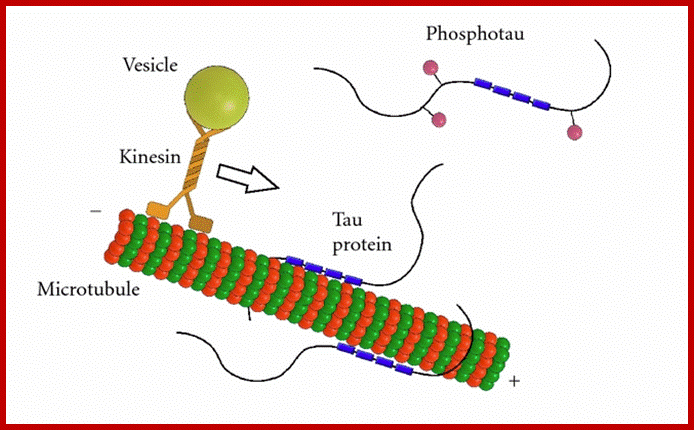
Tau protein: MT associated protein
Tau protein a dimer; https://dlcox.wordpress.com/
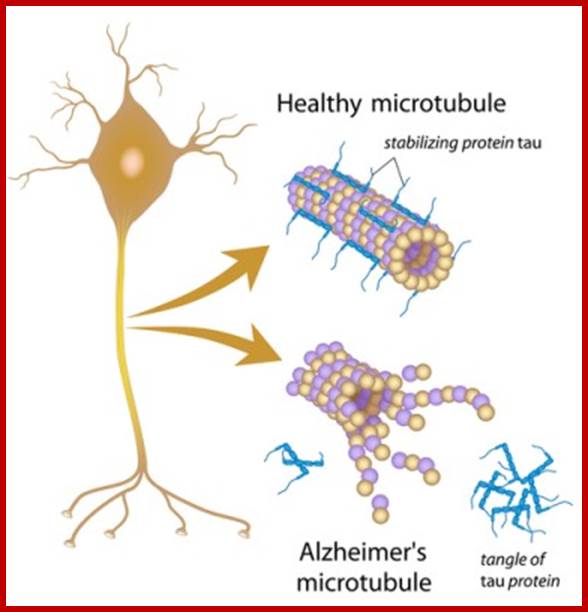
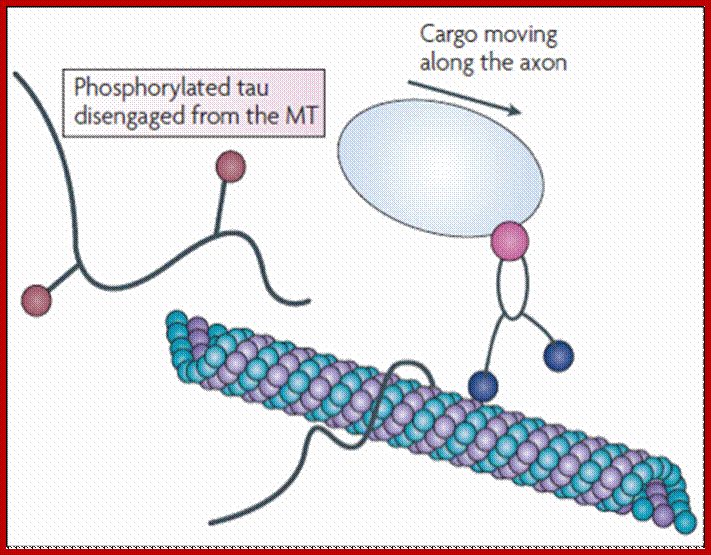
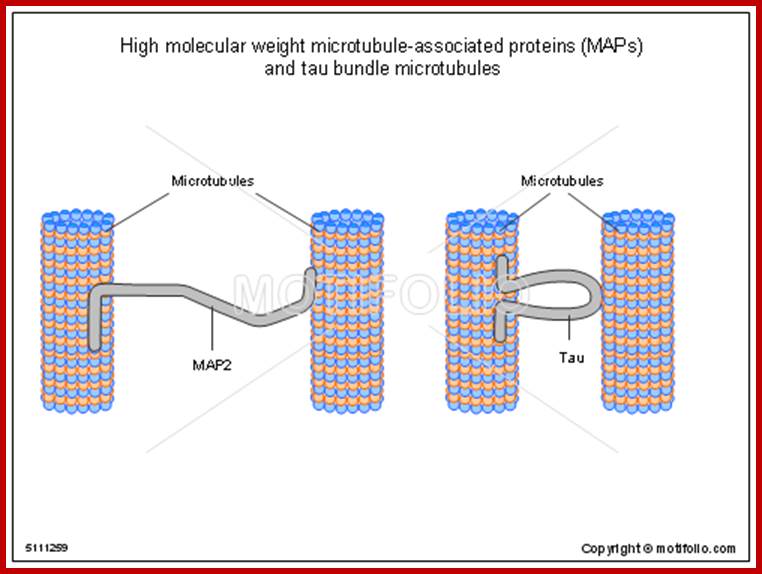
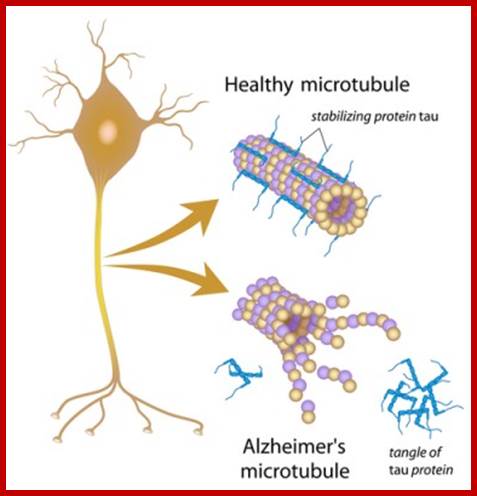
Tau proteins bind to microtubules through tubulin binding domains and stabilize them, Tau proteins are highly soluble and microtubule associated proteins (MAP). Tau binding facilitates their assembly and stabilization as well as motor-driven transport of axons Phosphorylation (pink) of tau can directly regulate its interaction with microtubules and its role in axonal transport. They have role in Alzheimer disease.
Its ability to stabilize microtubules is attributed to repetitive microtubule-binding motifs within its C-terminal region. These 18-amino acid repeat domains bind to microtubules through weak interactions such as van der Waals or ionic bonds. Specifically, the area 275-KVQIINKK-280 in the inter region between repeats 1 and 2 is highly potent in microtubule polymerization . Although the repeat domains are similar in structure, they differ in their binding affinities, which is likely attributed to slight differences in their amino acid sequence[. Their flexibility and lack of binding cooperatively further accentuate the protein’s transient secondary structure http://www.news-medical.net/ http://www.hindawi.com/; http://www.qstorm.org/ http://www.qstorm.org/
http://www.qstorm.org/
https://bionews-tx.com
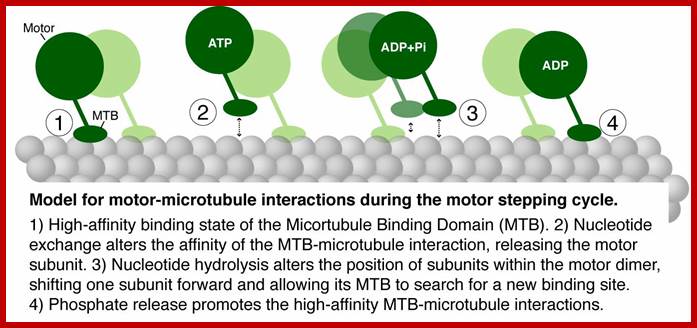
How do molecular
motors interact with microtubule tracks to generate motion?
We are conducting structure/function analyses of the microtubule track. The
carboxy-terminal tail regions (CTTs) of α- and β-tubulins contain an
abundance of negatively-charged residues thought to engage in electrostatic
interactions on the microtubule surface. These regions are major sites of
tubulin posttranslational modification and exhibit high sequence variation
amongst tubulin isotypes in humans. One compelling hypothesis is that CTTs
differentially affect motor interactions with the microtubule surface, thereby
regulating traffic at the level of the track. Consistent with this, we have
found that disruption of α- or β-CTTs elicits distinct defects in
microtubule function. We are currently characterizing these defects.
http://www.ucdenver.edu/
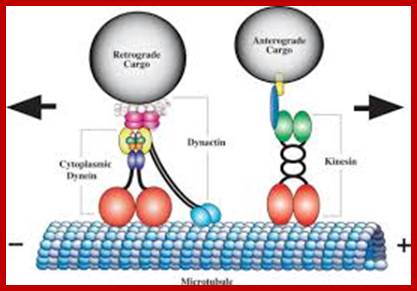
http://cellbiology.med.unsw.edu.aus
Functions:
Microtubules by themselves may have some functions, but in association with other cytoskeletal components they perform various functions such as mechanical support, vesicle movement, cell shape determination, organization of mitotic apparatus and chromosomal movement. The cytosol to remain semi viscous and mobile, microtubules provides the strength and fluidity. The cells to retain a particular shape require some mechanical support from within the cellular fluid and microtubules provide the same. The shapes of cells are determined by the inner organization of microtubular elements. These provide the basic skeleton system and organize into particular 3D order, thus they provide the mechanical support to the cell to maintain the shape of the cell.
Cell Elongation:
Auxin induced growth of plant cells is sensitive to colchicine, which indicates the involvement of microtubules in cell elongation. Actually, phytohormones activated membrane associated nucleating centers to assemble tubulin monomers into longer microtubules. The growth of a large number of such microtubules from associated nucleating is well documented. The growth of a large number of such microtubules which are oriented at the axis provides a mechanical force within the cytoplasm and makes the cell to stretch longitudinally. With loosening of the cell wall, deposition of enzymes and celluloses at the outer region of the PM facilitates the growth of cell condition with the cellulose enzymes the cell grows in length. Thus microtubules play a dynamic role in cellular growth.
Cell polarity fixation in morphogenesis:
The plane of cell division in the zygotes ultimately determines the polarity of stem apex and root apex. The light induced polarity fixation of the developing Fucus (Algae) zygote into rhizoid requires the presence of GTP as energy supply and Mg2 ions. In their presence alpha and beta tubulin subunits assemble on nucleating centers and grow into long tubular proteins basipetally i.e. from base to top. The assembly of tubulin monomers also requires microtubule associated proteins (map). In fact Maps play very important role not only in the assembly but also they are believed to control the orientation and alignment of microtubules within the cells
Motility:
Many unicellular algae contain two or more flagella or cilia as their motile organs. Such flagella or cilia are in fact made up of highly organized microtubular elements. They are also associated with motor protein components capable of hydrolysing ATP for the release of energy. Using such energy source the flagellar elements lash in water and bring about the movement.. They are also involved cytoplasmic streaming, where one can observe chloroplast movement. All most all forms of ER is supported by MTs. They perform in axons and dendrite area.
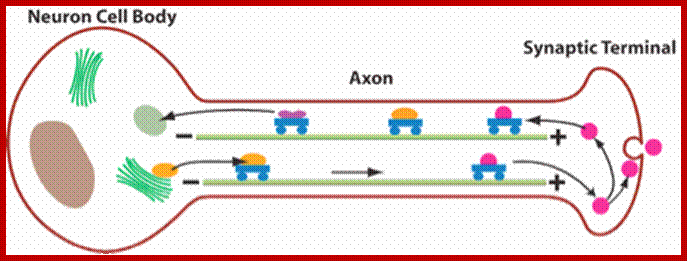
Cargos transported are golgi derived and endocytotic vesicles; they require specific motor proteins-such as Kinesins, Dyeins and Dynactins. The direction of Kinesins is always from minus end to Plus ends-called anterograde (200-400 mm/day) transport golgi derived vesicles, endocytotic and mitochondria and new cytoskeleton proteins and Dyenins is from plus ends to minus ends-called retrograde (200-300mm/day).
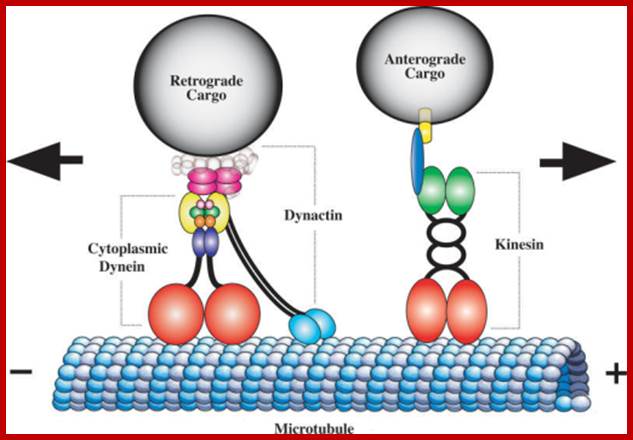
Dynein (+) to( -)end and Kinesins + end to – end, are motor
proteins move on microtubules in the direction mentioned, they are ATP
dependent; http://quizlet.com/;https://cellbiology.med.unsw.edu.au

The dynein intermediate chain (IC) is central to the structure of the dynein motor.2 It is composed of two domains. The extended N-terminal domain (N-IC), is indicated by grey solid and dotted lines. The C-terminal domain (C-IC), which interacts with the heavy chain, forms a relatively ordered and compact beta propeller structure indicated by the grey globular shapes in the figure. Two copies of IC are present in every dynein motor, and the dimer is bridged by the three light chains LC8, LC7, and Tctex-1. In addition to the light chains, N-IC contains interaction motifs for several other proteins known to be integral to the function of dynein. These include p150Glued, the largest subunit of the 'dynein activator' dynactin, and other proteins such as LIS1 and the ZW10 subunit of the Rod RZZ complex.
With its many interactions, N-IC appears to be the key modulator of dynein assembly and attachment to cargoes. With its extended structure, many interactions, and pivotal role in the function of a protein system, N-IC is representative of 'intrinsically disordered proteins'.3 The significance of this fascinating class of proteins has only recently been recognized. http://www.science.oregonstate.edu/
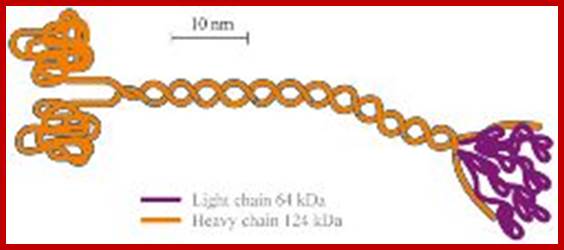
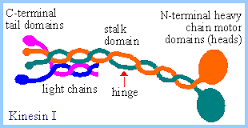
https://www.rpi.edu/dept
A kinesin molecule consists of two heavy chains whose molecular weight is ca. 120 kDa (thousand atomic mass units), and two light chains whose weight is 64 kDa. Heavy chains form globular motor domains at one end of the molecule, then they are twisted together to form a dimeric so-called coiled-coil domain. The opposite end of the kinesin molecule is formed by two light chains and is involved in the binding of cargo (vesicles, mitochondria). There are more than 14 known families of kinesin molecular motors. They mainly carry out transport from the centre of the cell in the direction of the cytoplasmic membrane. Motors which transport cargos along microtubules in the opposite direction belong to the family of dyneins. Kinesins, dyneins and other motor proteins use adenosine triphosphate (ATP) molecules as their energy source.; kinesins are a large family of proteins; Mammalian systems contain at least 40 kinesin genes; Kinesin I (conventional type) has a structure somewhat analogous to but distinct from that of myosin. There are 2 copies each of a heavy chain and a light chain. Each heavy chain includes a globular ATP-binding motor domain at the N-terminus. Stalk domains of the heavy chains interact in an a-helical coiled coil that extends from the heavy chain neck to the tail. The coiled coil is interrupted by a few hingeregions that give flexibility to the otherwise stiff stalk domain; N-termini of the two light chains associate with the two heavy chains near the tail. The diagram above is over simplified. Light chains at the N-terminus include a series of hydrophobic heptad repeats that are predicted to interact with similar repeats in heavy chains near the tail region, in a 4-helix coiled coil.
C-terminal tail domains of kinesin light chains include several "tetratrico peptide repeats" (TPRs). The 34 amino acid TPRs mediate protein-protein interactions. Kinesin light chain TPR repeats are involved in binding of kinesins to cargo. C terminal domains of heavy chains may also participate in binding some kinesins to cargo.
Cargo proteins bound by kinesins are diverse.
- Some organelle membranes contain transmembrane receptor proteins that bind kinesins. Kinectin is an endoplasmic reticulum membrane receptor for kinesin-I.
- Scaffolding proteins, first identified as being involved in assembling signal protein complexes, mediate binding of kinesin light chains to some cargo proteins or receptors.
- Some membrane-associated Rab GTPases, that provide specificity for vesicle transport and fusion, are known to bind particular kinesins.
http://eng.thesaurus.rusnano.com/ https://www.rpi.edu
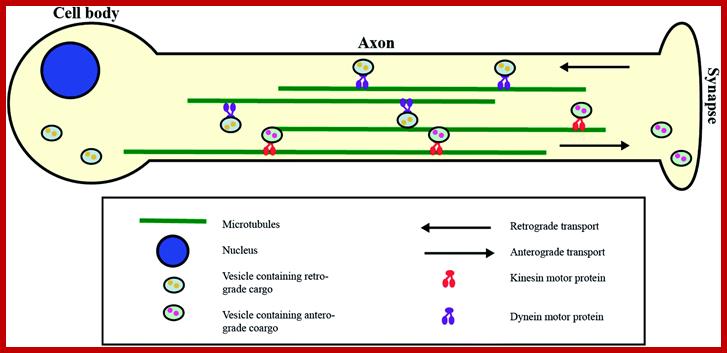
Transport across the length of Axon; http://pubs.rsc.org/
Structure of Dyneins and Dynactins;
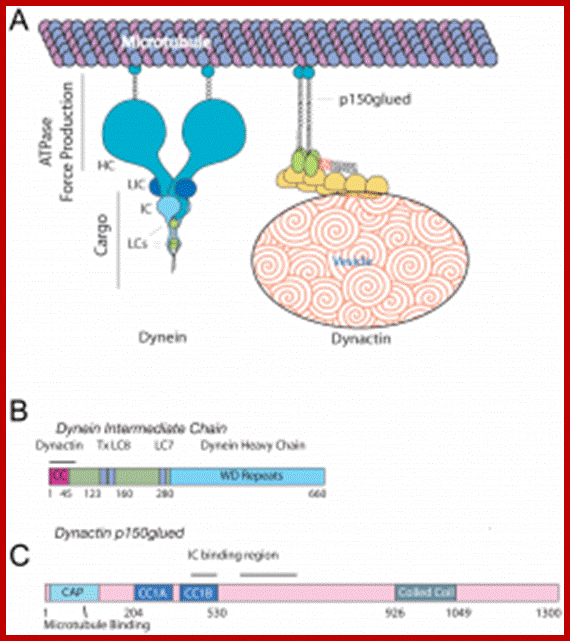
Structure of Dyneins and Dynactins; https://cellbiology.med.unsw.edu.au;
Dynein-and-dynactin-macromolecular-complex-organization-Both-complexes-are-drawn.png" alt="A. Dynein and dynactin macromolecular complex organization. Both complexes are drawn approximately to scale from EM reconstruction images. B. Schematic of domain structure and binding sites of both the dynein intermediate chain isoform 2C and dynactin p150Glued. The dynactin binding domain, spanning residues 10–44 (indicated by a black line), is within the predicted N-terminal coiled-coil domain of the IC, residues 1–45. The intermediate chain binding interface has been mapped to fragments spanning residues 217–548, denoted CC1, or 600–811 (indicated by a black line).
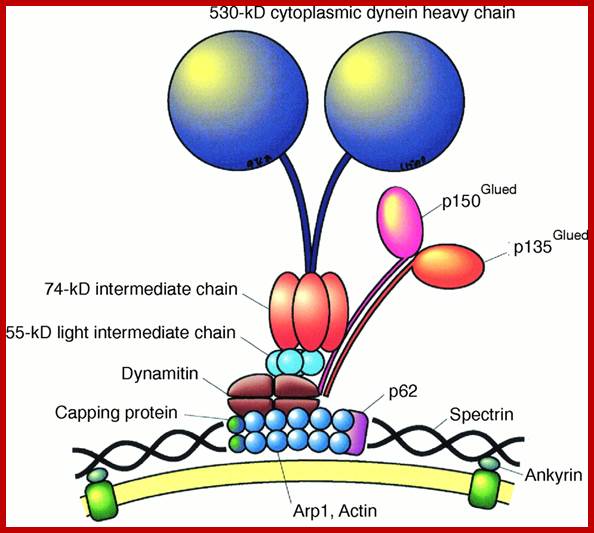
http://www.newmedicalterms.com/
Dynactins: They are multisubunit (ten 22-150kDa subunits) macromolecular complex, 1.2 MDa, they appear as short filaments of 37nm length and thin with globular heads. They have side arms that interact with Dynein; and an actin like minifilament that is bound to cargo. Dynactin is required for the binding of Dyneins to membrane vesicles including ER-to-Golgi, for transport, centripetal movement of lysosomes and endosomes, spindle formation, chromosome movement, nuclear positioning, and axonogenesis. Schematic of domain structure and binding sites of both the dynein intermediate chain isoform 2C and Dynactin p150 are Glued. The Dynactin binding domain, spanning residues 10–44 (indicated by a black line), is within the predicted N-terminal coiled-coil domain of the IC, residues 1–45. The intermediate chain binding interface has been mapped to fragments spanning residues 217–548, denoted CC1, or 600–811 (indicated by a black line).
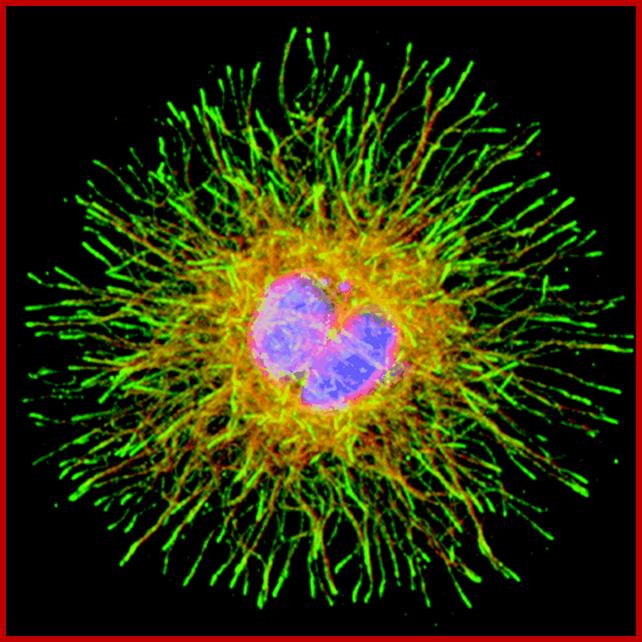
http://www.anti-agingfirewalls.com/
Cellular microtubule network; Microtubules are involved not only in axonal and dendrite transport but also involved in intracellular transport; which provides health and longivity
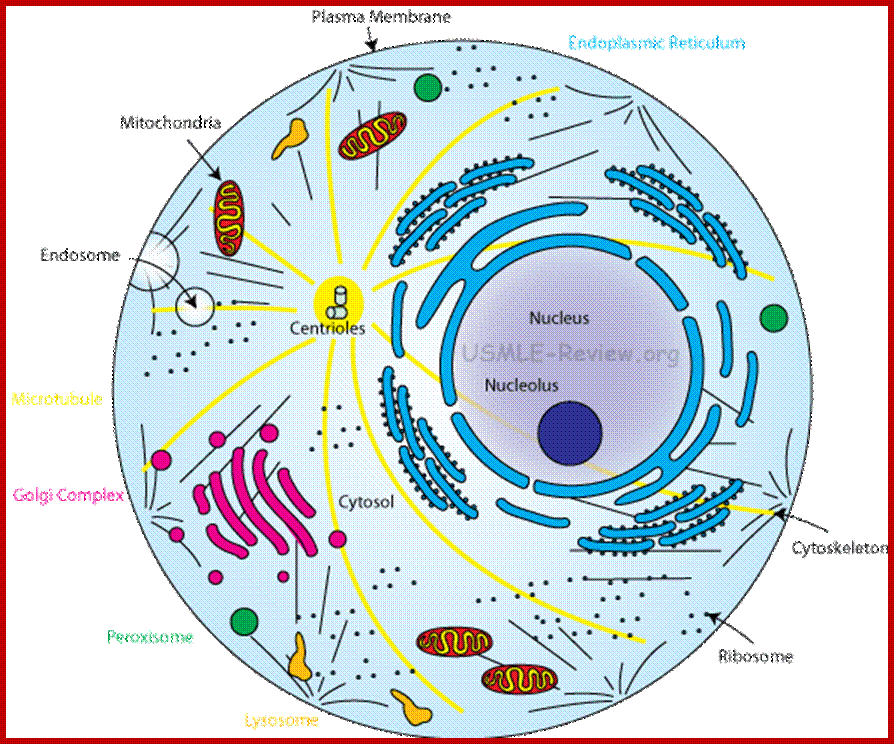
Centrosome; Region in the cell where microtubules originate; Contains centrioles. Plants lack centrioles; http://usmle-review.org/
.
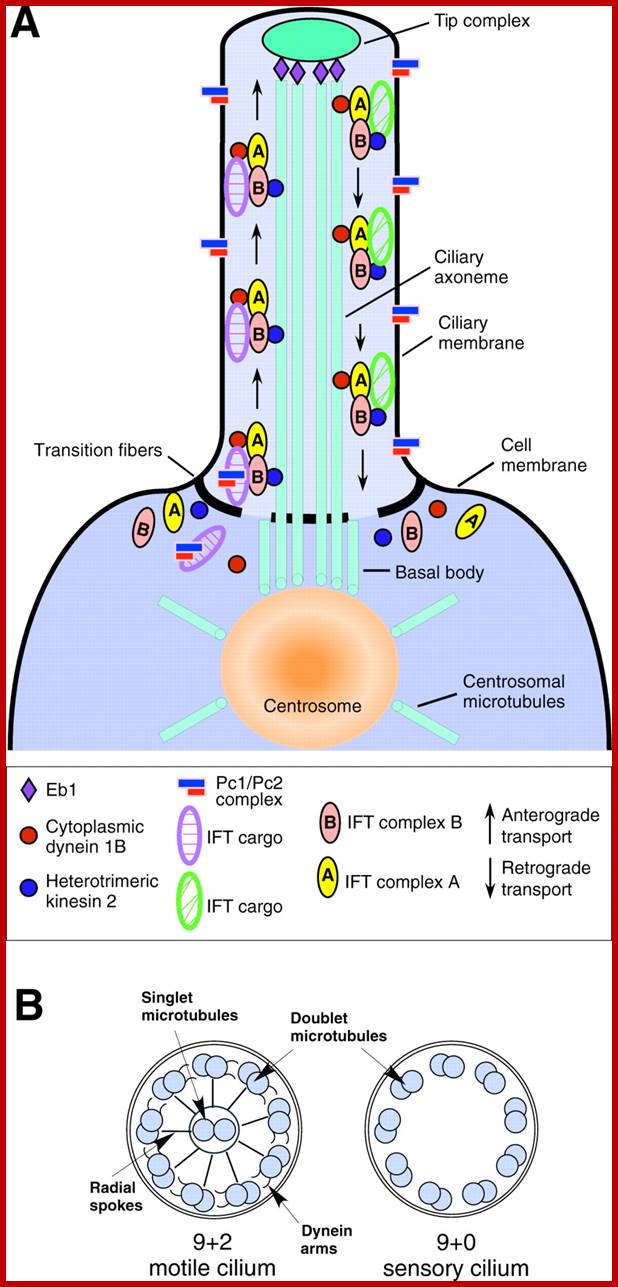
A cilium consists of an axoneme of nine doublets microtubules and each doublets arises from the inner two microtybules triplets. The axoneme is surrounded by a specialized membrane that is separated from the from the cell membrane by a zone of transition fibers. Coss section of 9+2 and 9=0 cilium; cilia are divide into 9+2 and 9+0 structures. Inner and outer dyneins arms which are attached to 9+2 cilia. Ciliary assembly and maintenance is accomplished by intra-flagellr transport (FT) which relies on MT motorproteins such as Kinesin2 and cytoplasmic Dynein t transport IFT protein complexes and their associated cargo up and down the length od cilium;
Microtubule as ciliary axoneme arising from basal body; Structure of the primary cilium; The core structure of primary cilia is composed of microtubule bundles (ciliary axoneme) extending from the basal body, a microtubule-based structure derived from the mother centriole. The ciliary membrane is continuous with the plasma membrane, but contains a unique protein composition, such as channels and receptors. Thus, primary cilia can function as a sensory organelle for receiving and transducing extracellular stimuli into cells, such as fluid flow or via signaling molecules. The inset image is of ciliated murine inner medullary collecting duct (IMCD3) cells in which the basal body is labeled with a γ-tubulin antibody (red) and the primary cilium is marked by an Ift88 antibody (green). http://dev.biologists.org/
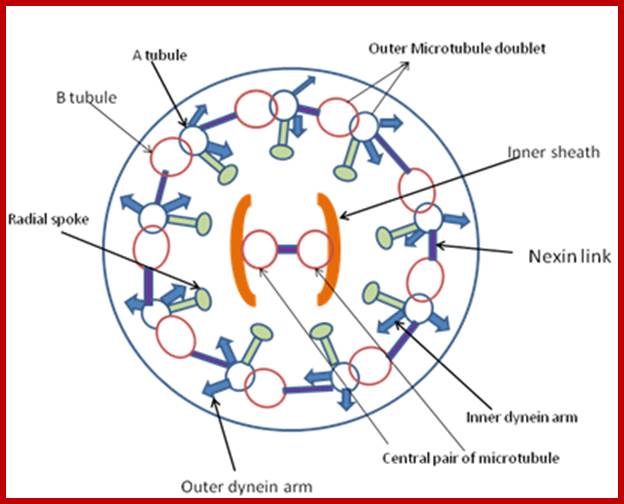
Axoneme arising from basal body; structure of axoneme of cilia and flagella. http://nptel.ac.in/
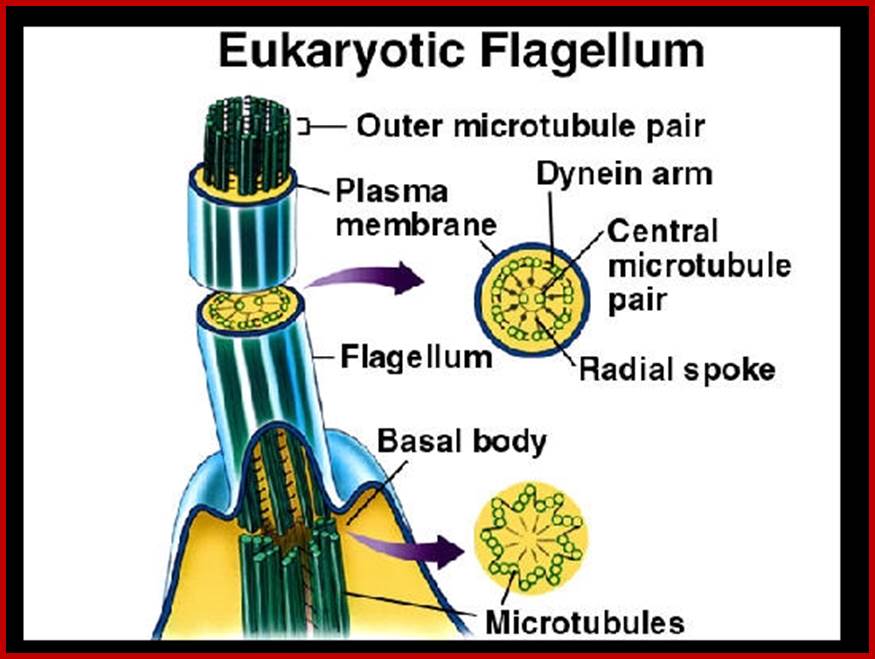
Organized MTs into flagella; www.thinglink.com
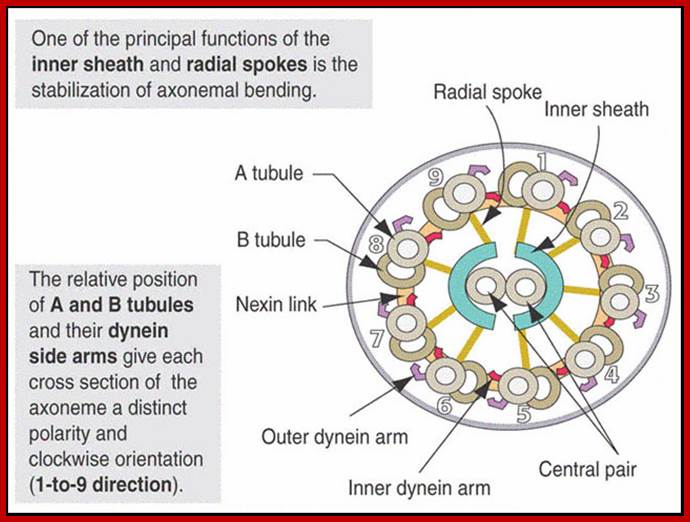
As the figure shows, there are nine paired microtubules surrounding two single microtubules. All of the ciliary structure that is inside the plasma membrane is called the axoneme. In addition to the microtubules, the cilia contain other proteins such as the dynein complexes, and several protein complexes that connect the outer microtubules to each other, and the outer microtubules to the inner microtubules. The structure is shown diagrammatically in the following figure. http://classes.kumc.edu/
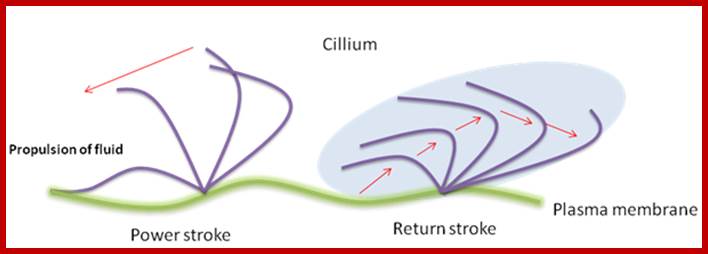
Ciliary and flagillar movement: The mechanism of force and movement (bending) by the flagellum has recently been studied extensively. It is well established now that the ciliary movement is generated by the microtubules and the associated structures of the flagellum. It was shown that the cell free flagella can be caused to move by adding an energy source such as ATP. Even broken pieces of cilia or isolated axoneme itself continue to beat, suggesting the role of microtubules in the movement. The contractile axostyle of some microorganisms such as Metamonadida. Bending force is produced by the sliding of microtubules.

Doublet centrioles duplicate and then develop into mitotic fibers (mitotic apparatus); www. explorebiology.com
Centrosome gives rise to mitotic spindle after duplication in animal cells; http://www.mskcc.org/
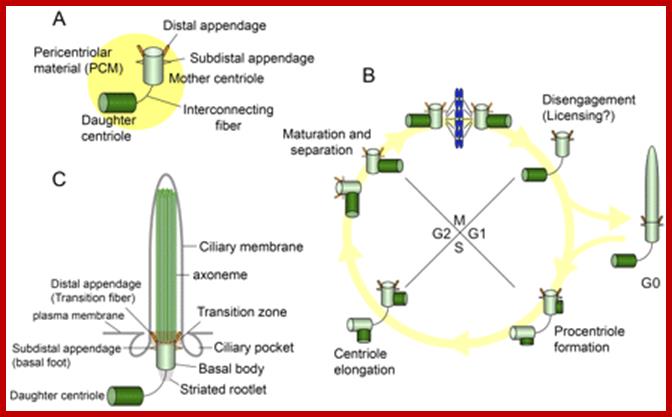
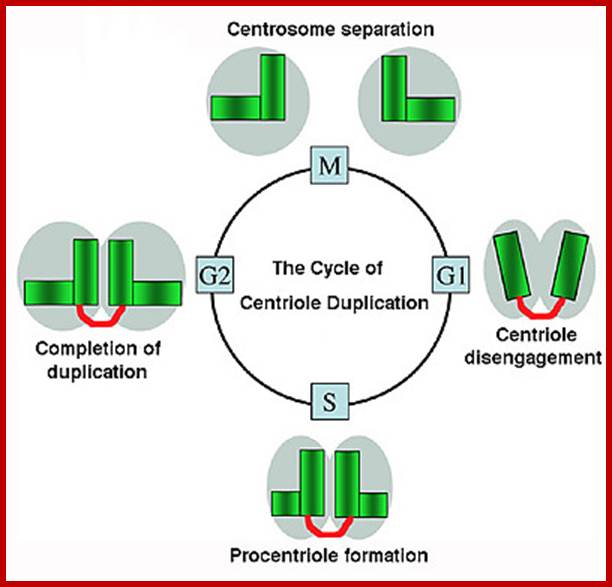
Centrosomes, cilia, and the cell cycle. (A) The centrosome is composed of mother and daughter centrioles, and a protein matrix called pericentriolar material. The mother centriole has distal and sub distal appendages. Interconnecting fibers connect the two centrioles. (B) New centrioles (procentrioles) assemble in S phase and continue to elongate in G2. The two paired centrioles separate, and the original (oldest) daughter centriole acquires appendage proteins in late G2/early M, although these appendages are not visible at this stage (appendages are depicted as dotted lines). After mitosis, the paired centrioles become disengaged. In G0, the mother centriole migrates near the plasma membrane to become a basal body, and the primary cilium is formed. (C) The basal body localizes near the plasma membrane and nucleates a primary cilium. The mother centriole converts to the basal body, and structures that include the transition fibers/distal appendage, basal foot/sub distal appendage, and striated rootlet are observed. The transition fibers tether the basal body to the plasma membrane in the transition zone, in which triplet microtubules of the basal body transition to doublet microtubules in the axoneme. The axoneme is surrounded by a ciliary membrane. Ciliary pockets are observed near basal bodies. http://jcb.rupress.org/
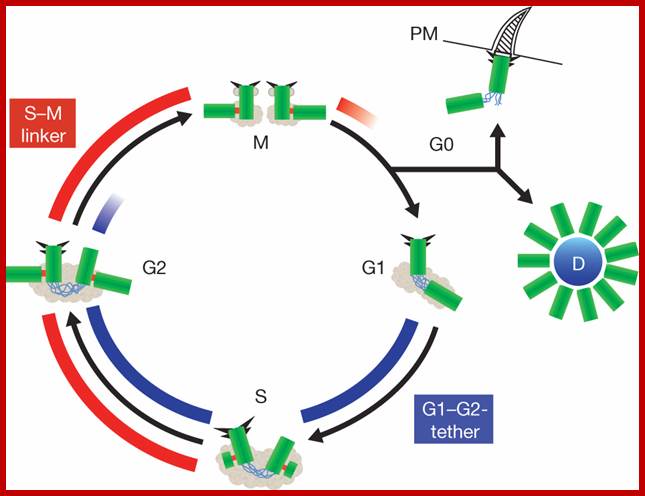
Centrosome Cycle: Centriole biogenesis, duplication and inherent asymmetries; Erich A. Nigg & Tim Stearns; www,nature.com
Centrosomes are microtubule-organizing centers of animal cells not in plant cells. They influence the morphology of the microtubule cytoskeleton, function as the base for the primary cilium and serve as a nexus for important signalling pathways. At the core of a typical centrosome are two cylindrical microtubule-based structures termed centrioles, which recruit a matrix of associated pericentriolar material. Cells begin the cell cycle with exactly one centrosome, and the duplication of centrioles is constrained such that it occurs only once per cell cycle and at a specific site in the cell. As a result of this duplication mechanism, the two centrioles differ in age and maturity, and thus have different functions; for example, the older of the two centrioles can initiate the formation of a ciliary axoneme. We discuss spatial aspects of the centrosome duplication cycle, the mechanism of centriole assembly and the possible consequences of the inherent asymmetry of centrioles and centrosomes. Nature Cell Biology http://www.nature.com/
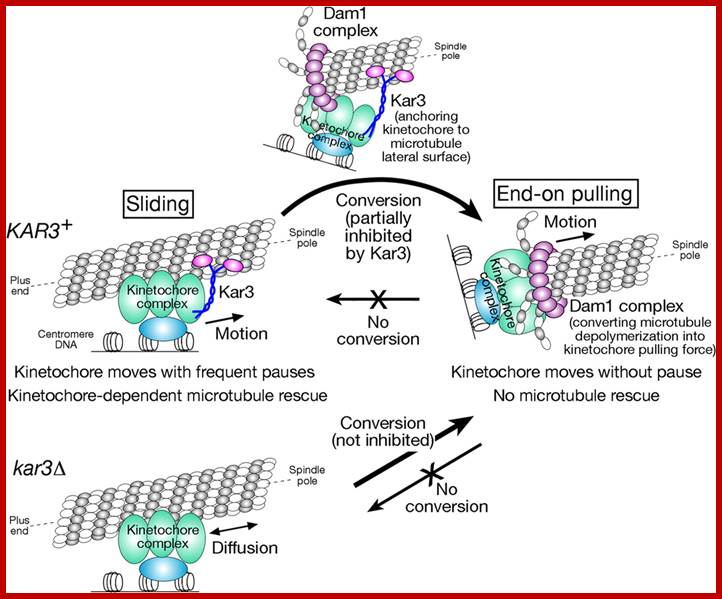
Molecular mechanisms of microtubule-dependent kinetochore transport toward spindle poles; Model for how kinetochores are transported by spindle microtubules. Poleward kinetochore transport occurs in two distinct ways: lateral sliding, in which kinetochores move along the side of a microtubule, and end-on pulling, in which the kinetochore is attached to the end of a microtubule and is pulled poleward as the microtubule shrinks. Kar3 is essential to drive poleward lateral sliding, whereas the Dam1 complex is crucial for end-on pulling. Kar3 partly suppresses the establishment of end-on pulling by anchoring kinetochores to the microtubule lateral surface. In the absence of Kar3 (kar3Δ), kinetochores show diffusion along the microtubule lateral surface. http://jcb.rupress.org/
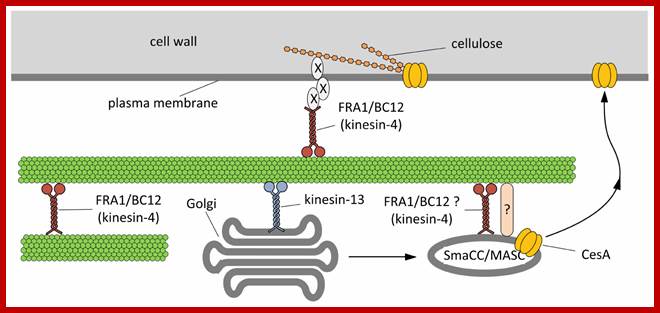
Hypothetical model outlining the functions of kinesins during the assembly of cell wall.On the basis of current literature, members of the kinesin-4 subfamily (FRA1 and/or BC12) might be used to organize microtubules beneath the plasma membrane in order to favor either the proper insertion or the activation of cellulose synthase (CesA). The interplay between kinesins and CesA might be also more direct. Members of the kinesin-13 subfamily have been hypothesized either to transport locally or to pause Golgi stacks along microtubules. Once assembled in the Golgi stacks, CesA might move into the so-called SmaCC/MASC compartments, which are known to interact with microtubules, a step required for the insertion of CesA into the plasma membrane. The proteins mediating the interaction between SmaCC/MASC and microtubules are partially known (indicated by question mark) and FRA1/BC12 might putatively be part of such complex. As a further hypothesis, FRA1 and BC12 might be part of the complex that organize the nascent cellulose microfibrils at the plasma membrane interface by delivering specific components that regulate the orientation of cellulose microfibrils (indicated by X). Giampiero Cai* and Mauro Cresti; http://journal.frontiersin.org/
Before prophase the microtubules form a ring in the equatorial plane of the cell (preprophase band), which disappears by the end of the phase. This is characteristic of the plant cells, but its function is not exactly known. Even if higher plants lack centrioles, they have cytocenters, which organize microtubules at the poles of the cell. The cytoplasmic organelles are distributed into the two halves of the cell. The mitotic spindle consisting of microtubules is formed from the two cytocenters; the elongating microtubules grow toward the chromosomes or toward the antipodal cytocenters. The former ones move chromosomes into the equatorial plane by joining to the kinetochore in the centromere region of the chromosome.
Before prophase the microtubules form a ring in the equatorial plane of the cell (preprophase band), which disappears by the end of the phase. This is characteristic of the plant cells, but its function is not exactly known. Even if higher plants lack centrioles, they have cytocenters, which organize microtubules at the poles of the cell. The cytoplasmic organelles are distributed into the two halves of the cell.
The mitotic spindle consisting of microtubules is formed from the two cytocenters; the elongating microtubules grow toward the chromosomes or toward the antipodal cytocenters. The former ones move chromosomes into the equatorial plane by joining to the kinetochore in the centromere region of the chromosome. During cytokinesis the vesicles coalesce, their pectinic content will form the cell plate, while their membrane will yield the new cell membranes of the daughter cells. At certain points the fusion is not complete; here develop the plasmodesmata connecting the cells.
Intracellular Transportation:
Protoplasm within the cells is in constant flux, which can be physically observed by the continuous movement of plastids and other organelles. Such protoplasmic movements can be brought to halt by treating the cells with colchicine or vinblastine. This further suggests the role of microtubules in intracellular transportation of materials. The association of microtubular elements with contractile microfilaments and microtrabaculae is very essential for the intracellular protoplasmic movements. The rate of such protoplasmic movements has been determined to be 20 mm – 30 mm per second. They are also involved in the transportation of vesicles loaded with enzyme components and required raw materials towards the plasma membrane for cell wall synthesis. The formation of pit canals, secondary cell wall thickenings are also determined by microtubular activities.
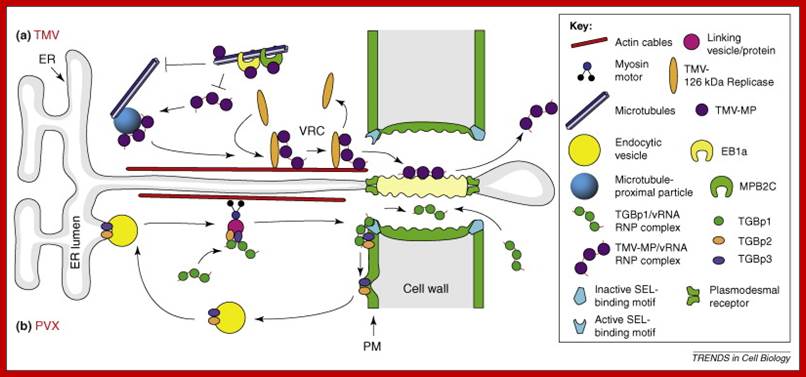
Plasmodesmata- in intercellular transport–bridging the gap between neighboring cells; www.cell.com
Microfilaments:
Microfilaments are another class of cytoskeletal proteins which exhibit contractile activity. Such filaments are associated with the plasma lemma and other endomembranes. They are found either as single elements or in-groups. In most of the cases, microfilaments act as cross linking bridges between microtubules. These structures also pervade the entire protoplasm. (Refer to proteins).
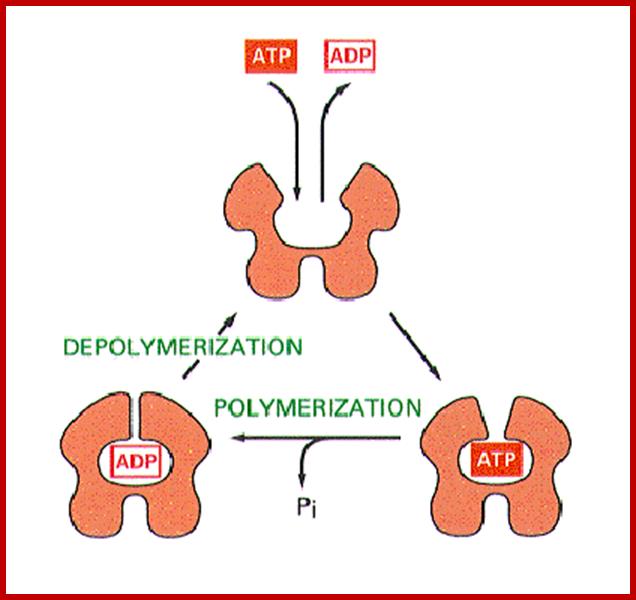
Actin is a globular protein with an ATP binding site in the center of the molecule. Termed "G-actin" the monomer will dimerize or form a trimer. This serves as a site for nucleation and further growth of the actin protofilament. Below each structure represents G-actin.
G-actin forms F-actin (the filament) in the presence of ATP, Mg and K. The concentration of G-actin is also critical. Above the critical concentration Cc of G-actin, the molecules will polymerize. Below the critical concentration, the actin filaments will depolymerize. ATP hydrolysis is not required for polymerization, but it is required to promote depolymerization (if it is converted to ADP). In this regard, it behaves like microtubules and their need for GTP hydrolysis to depolymerize. http://cytochemistry.net/
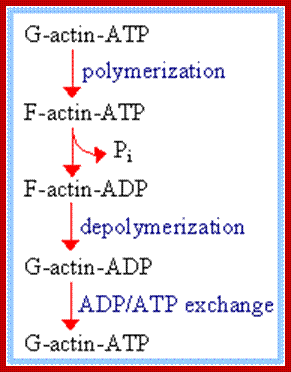
Acin filaments are made up of individual subunits called G-Actins. G-actin (globular actin) with bound ATP can polymerize, to form F-actin (filamentous actin).
F-actin may hydrolyze its bound ATP to ADP + Pi and release Pi. ADP release from the filament does not occur because the cleft opening is blocked.
ADP/ATP exchange: G-actin can release ADP and bind ATP, which is usually present in the cytosol at higher concentration than ADP.
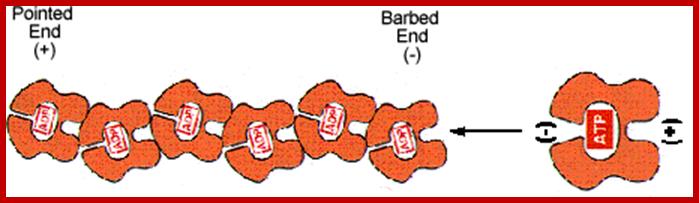
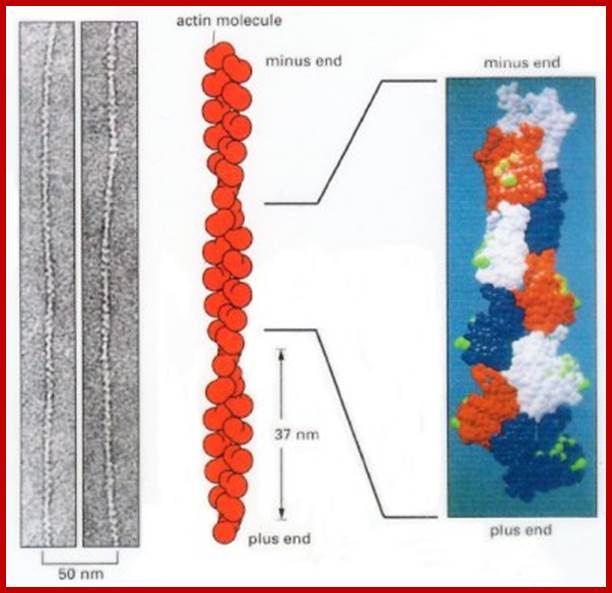
Actin monomers (G-actin) are polarized molecules, with a positive (+) “barbed end” and a negative (-) “pointed" end. The pointed end of G-actin-ATP binds the barbed end of another. The two remain attached after ATP is hydrolyzed. http://www.angelfire.com/
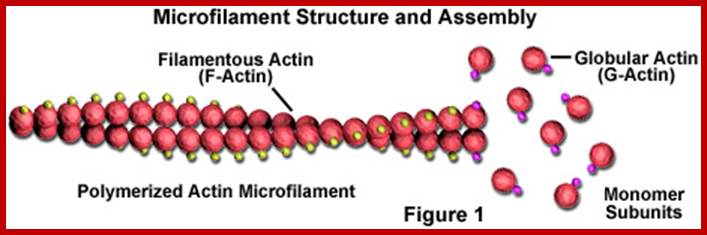
Microfilament structural organization; http://welkescience.wikispaces.com/
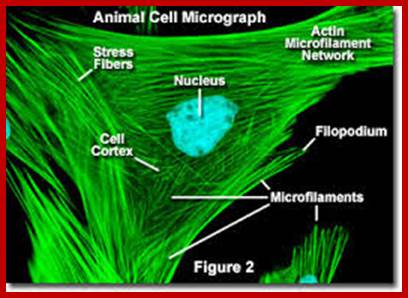
Microfilaments are solid rods made of a protein known as actin. When it is first produced by the cell, actin appears in a globular form (G-actin; see Figure 1). In microfilaments, however, which are also often referred to as actin filaments, long polymerized chains of the molecules are intertwined in a helix, creating a filamentous form of the protein (F-actin). All of the subunits that compose a microfilament are connected in such a way that they have the same orientation. http://welkescience.wikispaces.com/
The cytoskeleton of eukaryotic cells is continuously remodeled by polymerization and depolymerization of actin. Consequently, the relative content of polymerized filamentous actin (F-actin) and monomeric globular actin (G-actin) is subject to temporal and spatial fluctuations. Since fluorescence correlation spectroscopy (FCS) can measure the diffusion of fluorescently labeled actin it seems likely that FCS allows us to determine the dynamics and hence indirectly the structural properties of the cytoskeleton components with high spatial resolution. To this end we investigate the FCS signal of GFP-actin in living Dictyostelium disodium cells and explore the inherent spatial and temporal signatures of the actin cytoskeleton. Using the free green fluorescent protein (GFP) as a reference, we find that actin diffusion inside cells is dominated by G-actin and slower than diffusion in diluted cell extract. The FCS signal in the dense cortical F-actin network near the cell membrane is probed using the cytoskeleton protein LIM and is found to be slower than cytosolic G-actin diffusion. Furthermore, we show that polymerization of the cytoskeleton induced by Jasplakinolide leads to a substantial decrease of G-actin diffusion. Pronounced fluctuations in the distribution of the FCS correlation curves can be induced by latrunculin, which is known to induce actin waves. Our work suggests that the FCS signal of GFP-actin in combination with scanning or spatial correlation techniques yield valuable information about the local dynamics and concomitant cytoskeletal properties. Hanna Engelke, Doris Heinrich and Joachim O. Rädler; http://iopscience.iop.org/
Microfilaments function is that they provide
structural support for
the cell. Microfilaments are a
part of cellular cytoskeleton. Microfilaments are narrow tube like cell structures composed of proteins.
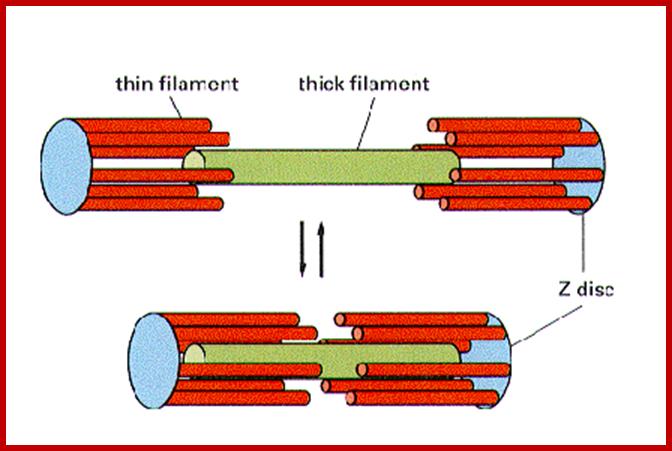
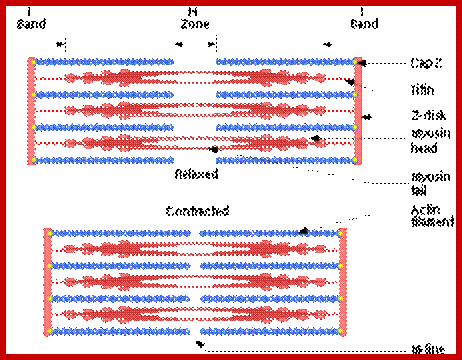
Sliding filament model of muscle contraction; http://en.wikipedia.org/
A cartoon of a sarcomere, showing the interactions is seen in the following figure. http://cytochemistry.net/
In the thick myosin filament, titan is used to provide elasticity and stability to the structure. Nebulin is used to stabilize the thin actin filaments. Actin is attached to the Z lines by alpha actinin.
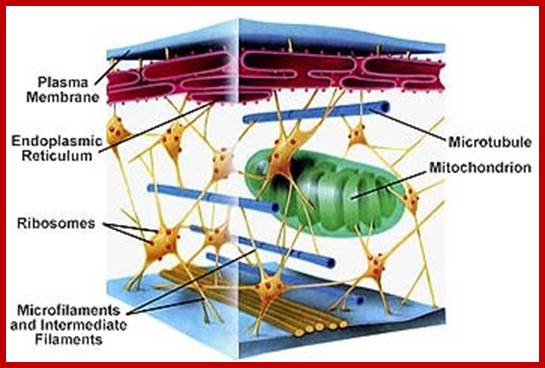
Organized ER, Microfilaments, Mcrotubules, Mitochondria and ribosomes in cytoplasm; www.studyblue.com
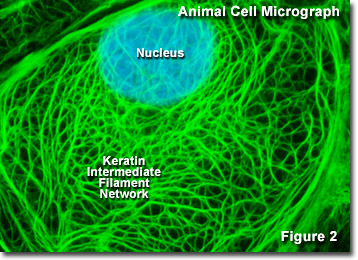
Keratin and intermediate filaments; micro.magnet.fsu.edu
Chemical Composition and Structure:
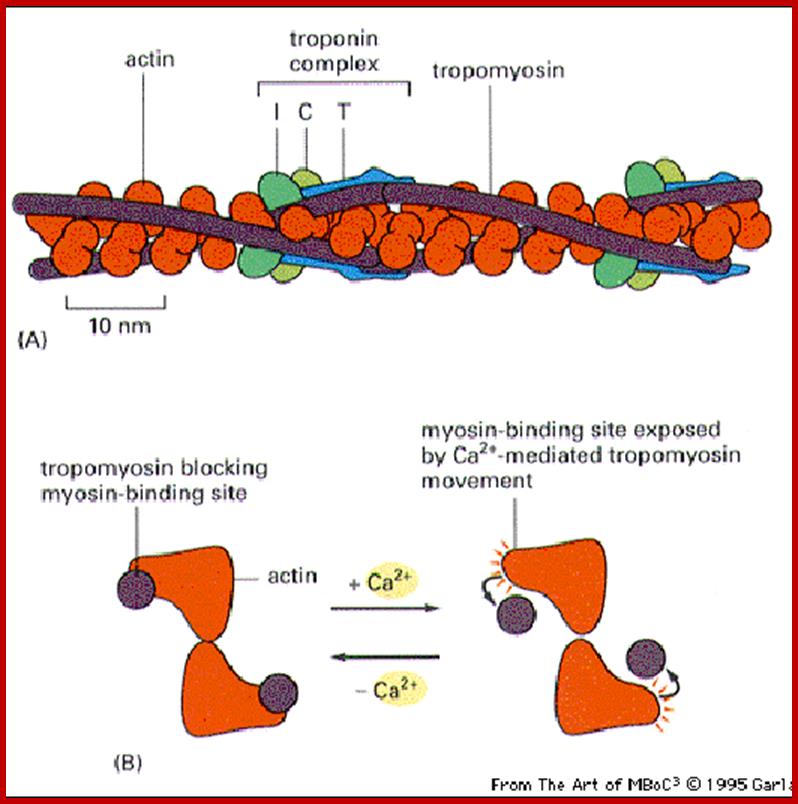
Microfilaments are basically made up of a number of globular protein subunits of mol. wt. 45 KD. Such units are called G actins. A large number of G actin units undergo polymerization in to fine filaments called F-actin fibers. Then two such filaments undergo relational coiling with each other with help of troponin, tropomyoin and some times myosin proteins to form functional microfilaments. They are highly sensitive to a drug called cytochalasin B, which prevents polymerization of G-units into F-actin; furthermore the microfilaments exhibit rapid changes from F-actin to G-actins. Microfilaments have been observed in various cell types, and pervasive. Because these are associated with ATP hydrolyzing components, microfilaments exhibit contractile activity.
Functions:
It should be remembered that microfilaments are associated with other cytoskeleton elements and their function depends upon the co-coordinated association and function of other elements. However they perform a variety of functions like protoplasmic steaming, pseudopodial movements, contraction and others.
Cytoplasmic Cleavage:
When cells stained with fluorescent dyes and observed under fluroscense microscopes, the microfilaments and microtubules are found to be organized into array of filaments associated with cell membranes. Infect the position of these filaments with respect to membranes can be disturbed and they can be induced move laterally by treating the cells with proteins such as Lectins. However, during cleavage, the microfilaments and microtubules associated with membranes at the cleavage site contract and the membrane is drawn inwards in the form constriction. By further contraction inwards, the cytoplasm gets cleaved into two. A similar situation is observed during endocytosis and phagocytosis.
Chromosomal Movements:
Kinetochores found in the centromeric regions of chromosomes develop long tractile fibers during metaphase of mitosis and meiosis. The microtubular elements of tractile fibers are cross linked with actin fibrils. So also spindle fibers are microtubular elements. The association of contractile actin filaments brings about sliding movements similar to muscle proteins. Thus microfilaments greatly facilitate the chromosomal movements towards their respective poles.
Protoplasmic streaming:
Rapid movement of protoplasm within the cells of Elodea, staminal hairs of tradescantia, nietella, chara and other cells is often referred to as protoplasmic streaming. Such movements are stimulated by light, chemicals and phytohormones. But the same can be inhibited to greater extent by the treatment of cells with cytochalasin B which is known to inhibit polymerization of G actions into F actin filaments. Electron microscopic studies reveal the presence of bundles of microfilaments and microtubules at the cytosolic surface of plasma membranes. Recent studies indicate that the protoplasmic streaming is mainly controlled by rapid contractile activity of microfilaments and the pathway within the protoplasm is determined by microtubules. Rapid interconversions of sol into gels and vice versa create intracellular cytoplasmic streaming which may of rotational or cyclosis type.
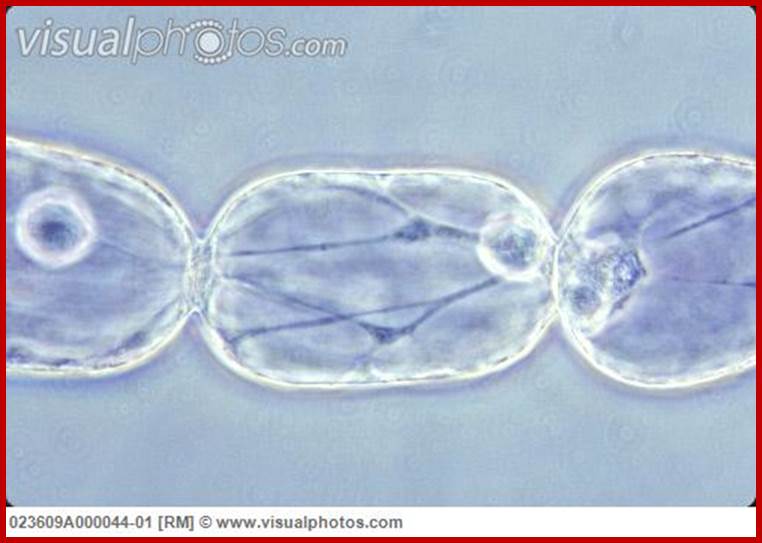
Tradescatia staminal hairs- showing protoplasmic streaming; http://www.visualphotos.com/
Cytochalasin B treatment of protoplasts and other animal cells brings about the extrusion of nucleus. However mechanism, by which microfilaments deploymerization brings about the extrusion of nucleus, is not clear.

Plant formins: Membrane anchors for actin polymerization;
In plants, the actin cytoskeleton plays a fundamental role in intracellular transport, cell growth, and morphology. Formins are central regulators of actin polymerization and actin-based processes in many eukaryotes. Plants have a diverse family of formins and this diversity arose early in land plant evolution, probably deriving from family expansion and domain acquisition. Recently, formins from different plant lineages have been studied and the focus of these studies is beginning to shift from biochemical characterization to in vivo function. In vivo studies have shown that distinct biochemical activities confer specific cellular functions. Despite these differences, many plant formins have in common a direct link to the plasma membrane, suggesting that formins in plants are important links between the plasma membrane and actin remodeling. Peter A.CVan Gisbergen, Magdalena Bezanilla;www.cell.com
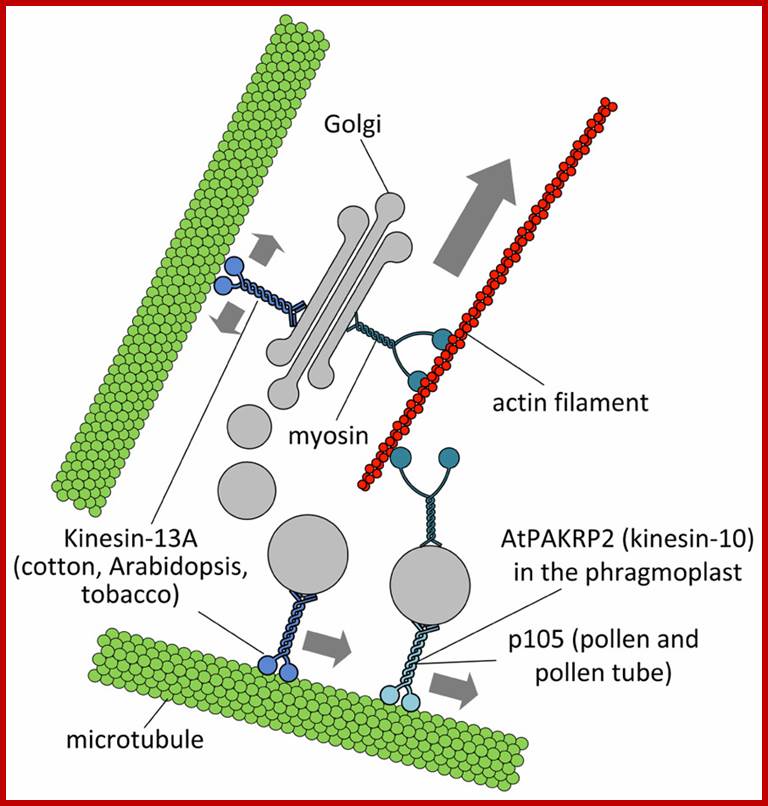
Putative interactions between Golgi stacks/vesicles and motor proteins. Specific subclasses of myosin XI would be responsible for the long-range transport of Golgi bodies within plant cells. Members of the kinesin superfamily are likely involved in either the anchorage of Golgi stacks to microtubules (such as kinesin-13A) or the short-range transport of Golgi vesicles to defined cell positions (such as AtPAKRP2 and p105). The arrow length indicates the relative velocity as produced by motor proteins. Opposing arrows indicate an anchorage role for the motor. http://journal.frontiersin.org/
Microtrabaculae.
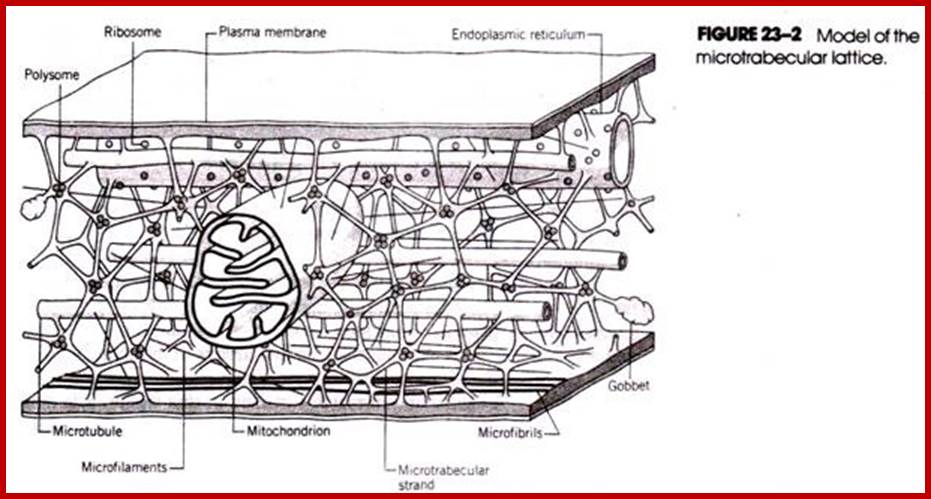
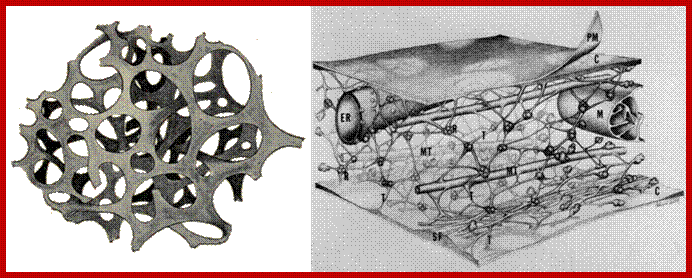
The presence of microtubules and microfilaments in cellular protoplasm is known in the past. But recently another structural element has been visualized with the help of high voltage microsocpy. This structure is called microtrabaculae. Keith Porter proposed (1970) the presence of another class of cytoskeleton structures. The network of this protein complex is spread throughout the cytoplasm. The interspaces found within such a network is about 3-6nm (50-100 nm?) which is large enough for the rapid diffusion of fluids and metabolites to all parts of the cytosol. The microtrabacular lattice is not rigid structure. Rather it varies in response to changes in cellular activities and shape of the cell. Its disposition in a cell also varies according to the cellular environment. The only structure that is free from the ramification of all pervasive microtrabaculae is mitochondria. It is difficult to imagine why only this organelle is free form the ramification of such structures.
http://www.biologydiscussion.com/
http://biochemistri.es/
Reincarnation of microtrabacular lattice; The Axoplasm is cytoplasmic constituent and it migrates to a length of 400mm/day or at the rate of 5microns /sec. The axoplasmic elements are microtubules and neurofilaments are interconnected and elongated varicose components of smooth ER dilated vesicular organelles (resembling SER) and multi-vesicular bodies , mitochondria and matrix as ground substance in which tubules, filaments and vesicles are combinatorial suspension; all this forma a 3-D lattice. This is microtrabacular matrix. Such structures were made possible by special techniques
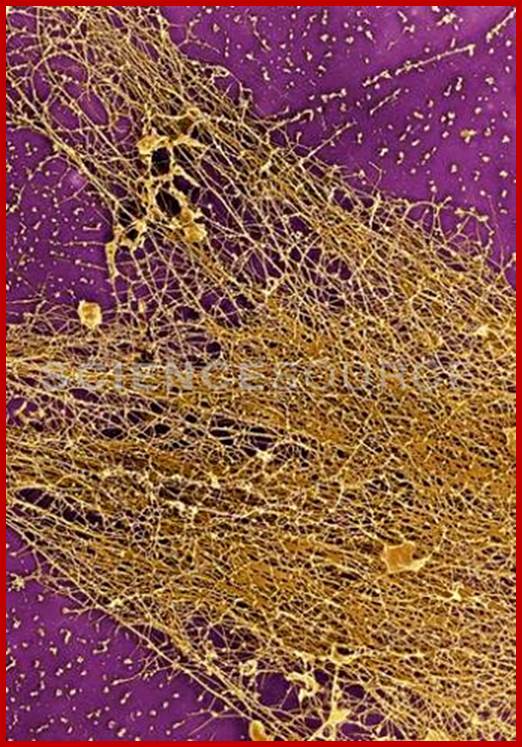
Scanning electron micrographs and the high resolution of transmission electron microscopy. http://images.sciencesource.com/
The microtrabacular lattice apparently serves as an intracellular scaffolding that helps suspend and ‘ organize the diverse structural components of the cytoplasm, including many of the cellular organelles. Acting in concert with the cytoplasmic filaments and microtubules, the lattice plays an important role in maintaining cell shape and in cellular movements. Existence of such structures has been subjected controversy for some scientist believe there is no such structure for the cytoskeletal elements when together appear as microtrabaculae.
Chemical Composition and Structures:
Isolation of microtrabacular network free from other structures was a formidable task but biologists have succeeded in isolating such structures at least in crude form. The analysis of protein components of such elements indicates the presence of tubulin and actin protein subunits. But the 3-D view of microtrabaculae reveal the presence of microtubules, microfilaments as well as smaller microtubules of 50-80 dimensions. Such filaments are shorter than other normal microtubules; they form a network by themselves by interacting with each other by their dichotomous ends. They actually appear as ultramicroscopic spicules held to each other to form a network. In fact such structures at the intersections of spicules where two or three tubules meet each other a group of polyribosomal complexes are found. And ribosomes are engaged protein synthesis free from ER. But the same structures are also associated with larger microtubules all over their surface.
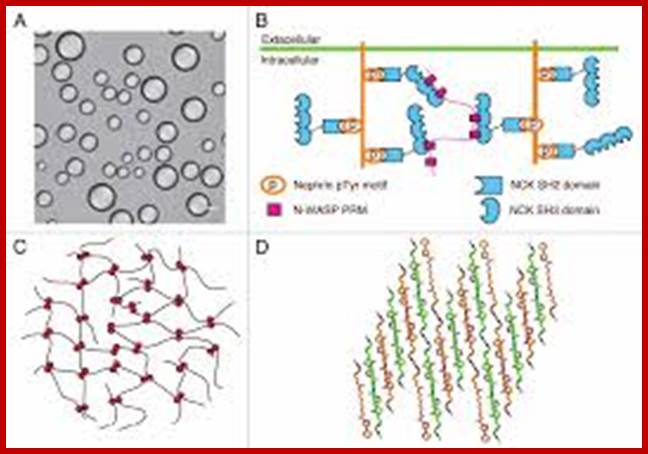
A. Liquid droplets from multivalent SH3-PRM (NCK/WASP) system; B. Nephrin; http://www.tandfonline.com/
By the limited evidence available to date, this phase transition phenomenon seems conceptually connected with the extinct MTL concept. Whereas today it is considered as an artifact of fixation, it was thought to arise via cross-linking of elements of cytoskeleton (microfilaments and intermediate filaments) and proteins in their proximity, perhaps already in loose association with them. It is of note that several hydrogel-forming IDPs are related to the cytoskeleton (actin regulatory proteins and neurofilament side-arms). That is, even if the MTL system as suggested decades ago does not exist, distinct yet coherent observations of phase transitions of repetitive disordered IDPs/IDRs calls for a revival/extension of our concepts of the physical inhomogeneity of cytoplasmic constituents. The long-gone concept of the microtrabecular lattice calls for appropriate approaches for their visualization and characterization in living cells, in order to uncover thus far unappreciated physical means of regulation of cell physiology
The microtrabacular microtubules are also sensitive to low temperatures and colchicine. They also exhibit rapid assembly and disassembly. They are more mobile than larger microtubules. Association of such tubules with contractile components gives a greater dynamics which facilitates rapid interconversions within the cytoplasm.
The 3-D structure as visualized by high voltage electron microscopy reveals the pervasive nature of smaller microtubular elements associated with larger microtubules, bundles of microfilaments, endoplasmic membranes and polyribosomal complex. Such tubules are in contact with all organelles with the exception of mitochondria. They also exhibit gliding movements over the surface of microtubules.
Functions:
Some of the functions attributed to microtrabacular network are over lapping with the functions performed by other partners of cytoskeletal fabrics like microtubules and microfilaments. Hence it is difficult to pinpoint the exact role of microtrabaculae per se. studies on the behavior of cells of Actinosphaerium, melanocytes, and fish erythrophores demonstrate that microtubules provide surfaces for the movement of materials, over their surfaces. It is on the surface of microtubules, various components like cytoplasmic granules, melanin and other pigments particulates move over to the form clusters in response to various external stimuli. The microtrabacular filaments further show very rapid gliding movements over such microtubular elements. By such observations, it has been deduced that these are very important cytosolic components engaged in rapid intracellular transportation.
Similar studies on sieve tube elements found in higher plant vasculatures reveal the presence of longitudinally oriented microtubules whose surfaces are covered by a large number of smaller microtubular elements. The active transportation of sucrose and other organic components through sieve tubes is attributed to the functions of such structures called microgels.
Cytoskeleton elements are also responsible for positioning mRNA at specific position in the cell. In developing Drosophila egg in to an embryo mRNA produced maternally are transported and positioned at specific poles; such transportation is aided by microtubules and microfilaments assisted by motor proteins.
Protein Synthesis:
The site of protein synthesis on rough endoplasmic reticular membranes is a fact. But the protein synthesis outside endomembranes is believed to be due to free polyribosome-mRNA complex activity. Now it is known the free cytosolic protein synthesis actually takes place at the intersection of microtrabacular and actin network. However the entire composition has not well worked out. The polysomal complexes with their mRNA are active only when they are associated with microtrabacular elements. If they are freed from such network, the protein synthesis of such polysomal complexes is inhibited. This view has been supported by some observations where certain animal viruses, after infecting the host cells, inhibited so called free polyribosome mediated protein synthesis. Now it has been demonstrated that viral mRNAs displace host cellular mRNA associated polyribosomes found at the intersection of microtrabacular network, and start synthesizing the viral proteins. So, for the time being, it can be concluded that microtrabaculae perform a variety of functions within the cells. Presently the existence of microtrabecule has been doubted and people consider this as the artifact of electron microscopy; it is hard to believe such things in science.
Intermediary filaments:
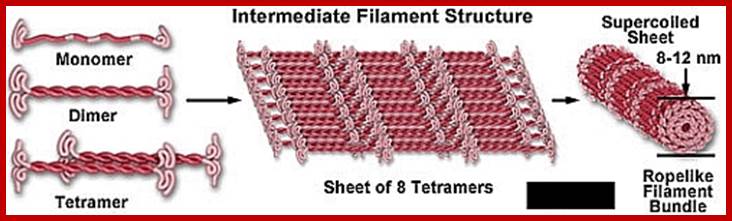
Different forms of Intermediate filaments; www.studyblue.com
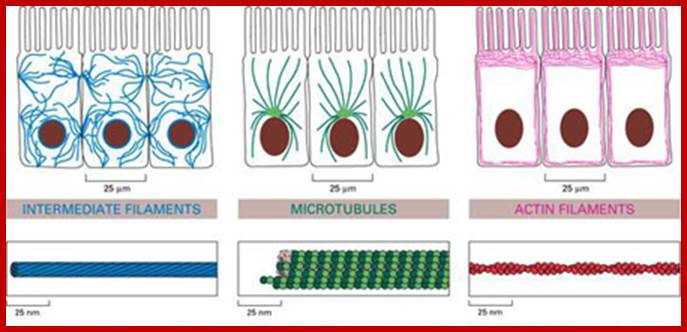
Intermediate filaments are important components of the cell's cytoskeletal system. They may stabilize organelles, like the nucleus, or they may be involved in specialized junctions. They are distinguished from "thin filaments" by their size (8-10 nm) and the fact that thin filaments are obviously motile. However recent evidence indicates that Intermediate Filaments may also have dynamic properties. Intermediate filaments are one of three types of cytoskeletal elements. The other two are thin filaments (actin) and microtubules. Frequently the three components work together to enhance both structural integrity, cell shape, and cell and organelle motility. Intermediate filaments are stable, durable. They range in diameter from 8-10 nm (intermediate in size compared with thin filaments and microtubules). They are prominent in cells that withstand mechanical stress and are the most insoluble part of the cell. The intermediate filaments can be dissociated by urea.
There are five different types of Intermediate filaments:
- Types I and II: Acidic Keratin and Basic Keratin, respectively. Produced by different types of epithelial cells (bladder, skin, etc).
- Type III. Intermediate filaments are distributed in a number of cell types, including: Vimentin in fibroblasts, endothelial cells and leukocytes; desmin in muscle; glial fibrillary acidic factor in astrocytes and other types of glia, and peripherin in peripheral nerve fibers.
- Type IV Neurofilament H (heavy), M (medium) and L (low). Modifiers refer to the molecular weight of the NF proteins. Another type IV is "internexin" and some nonstandard IV's are found in lens fibers of the eye (filensin and phakinin).
- Type V are the lamins which have a nuclear signal sequence so they can form a filamentous support inside the inner nuclear membrane. Lamins are vital to the re-formation of the nuclear envelope after cell division.
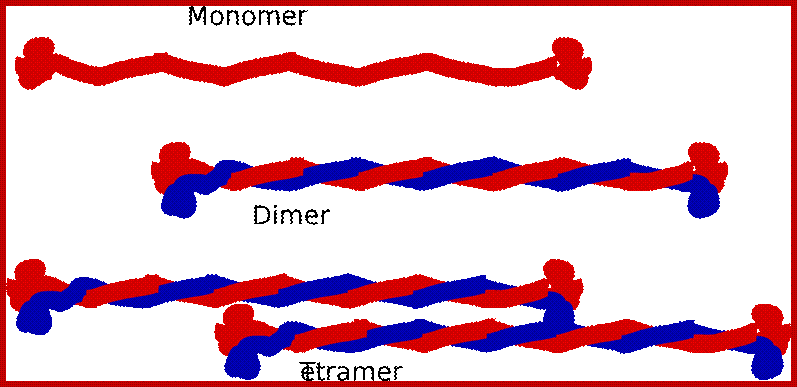
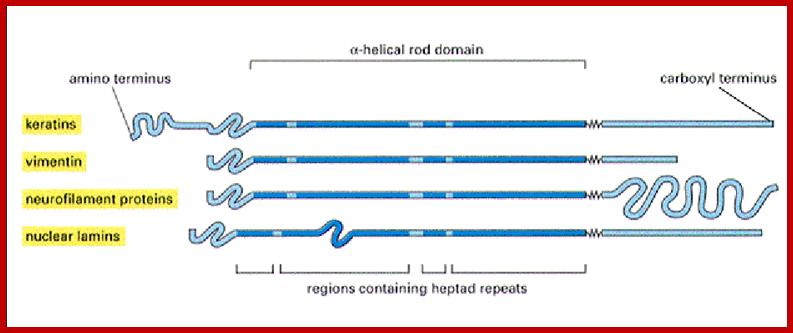
5. The monomer:
6. Each intermediate filament monomer consists of an alpha helical rod domain, which connects the amino (head) and carboxyl (tail) terminals. The figure below (16-12 from Albert’s et al Biology of the cell, Garland Publishing, N.Y. 1996) shows some examples of monomers.
Formation of the protofilaments:
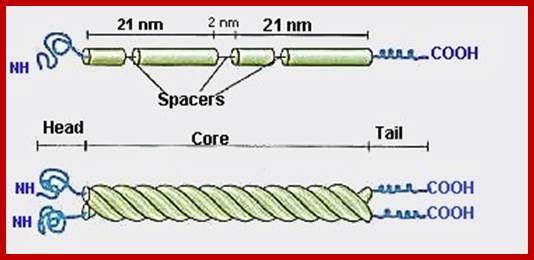
http://cytochemistry.net/
he rods coil around another filament like a rope to form a dimer. The N and C terminals of each filament are aligned. Some Intermediate filaments form homodimers; other form heterodimers.
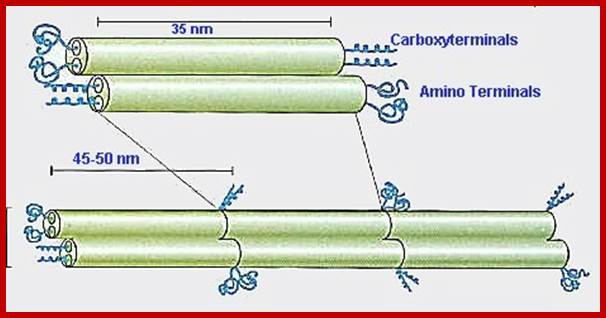
http://cytochemistry.net/
These dimers then form staggered tetramers that line up head-tail. Note that the carboxy and amino terminals project from this protofilament. This tetramer is considered the basic subunit of the intermediate filament.

http://cytochemistry.net/ The final 10 nm filament is a helical array of these tetramers.
Regulation of Assembly or disassembly of intermediate filaments:
Most of the intermediate filaments are fully polymerized. However, there is evidence that even these stable structures have dynamic properties. There is some free tetramer in the cytoplasm as if this is the basic subunit for assembly of new filaments. Also, if one phosphorylates serine residues in the amino terminals one can cause disassembly.
Add labeled tetramer to a cell that produces that type of intermediate filament. Can watch the tetramer become incorporated into the cytoskeletal system and the label is seen in a linear or squiggle array. If you add it to a cell that does not normally produce the tetramer, then it will not add and the cytoskeletal system will not "light up".
This can be tested by Fluorescence recovery after photo bleaching (FRAP).
This technique uses UV laser light to bleach an area of label in a cell. Then, one can time the recovery of the label either following introduction of new labeled material, or via simple movement of label into the structure. In the case of the Intermediate filaments, FRAP allows one to detect incorporation of labeled tetramers into a bleached spot in the cytoskeleton. One can compare times of incorporation of different types of tetramers into different types of Intermediate filaments. One can also observe motility of these structures. The paper that will be read for this lecture shows examples of both of these types of tests. In the cell below, a dark spot forms after laser photo bleaching. The spot is smaller after 30 min and almost gone after 2 h.
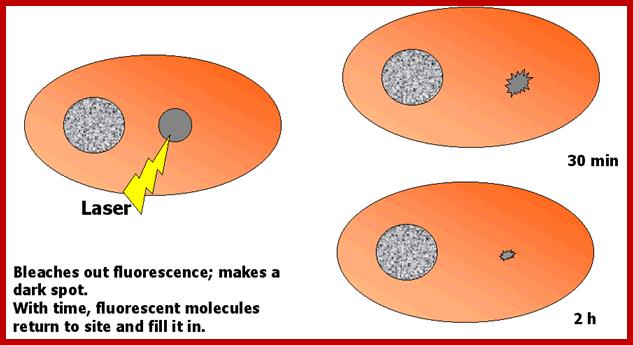
http://cytochemistry.net/
Intermediate Filament Associated Proteins;
Intermediate filament associated proteins may bind filaments in cross linked (to improve stability), or they may bind the filaments to other structures. Some examples are seen below.
· Plectin: Cross links with microtubules
· Lamin receptor B: binds to inner nuclear membrane
· Ankyryn: binds actin to Intermediate filaments at base of cell
· Desmoplakin: binds Intermediate filaments at site of desmosome
he rods coil around another filament like a rope to form a dimer. The N and C terminals of each filament are aligned. Some Intermediate filaments form homodimers; other form heterodimers.
The final 10 nm filament is a helical array of these tetramers.
Regulation of Assembly or disassembly of intermediate filaments:
Most of the intermediate filaments are fully polymerized. However, there is evidence that even these stable structures have dynamic properties. There is some free tetramer in the cytoplasm as if this is the basic subunit for assembly of new filaments. Also, if one phosphorylates serine residues in the amino terminals one can cause disassembly.
Add labeled tetramer to a cell that produces that type of intermediate filament. Can watch the tetramer become incorporated into the cytoskeletal system and the label is seen in a linear or squiggle array. If you add it to a cell that does not normally produce the tetramer, then it will not add and the cytoskeletal system will not "light up".
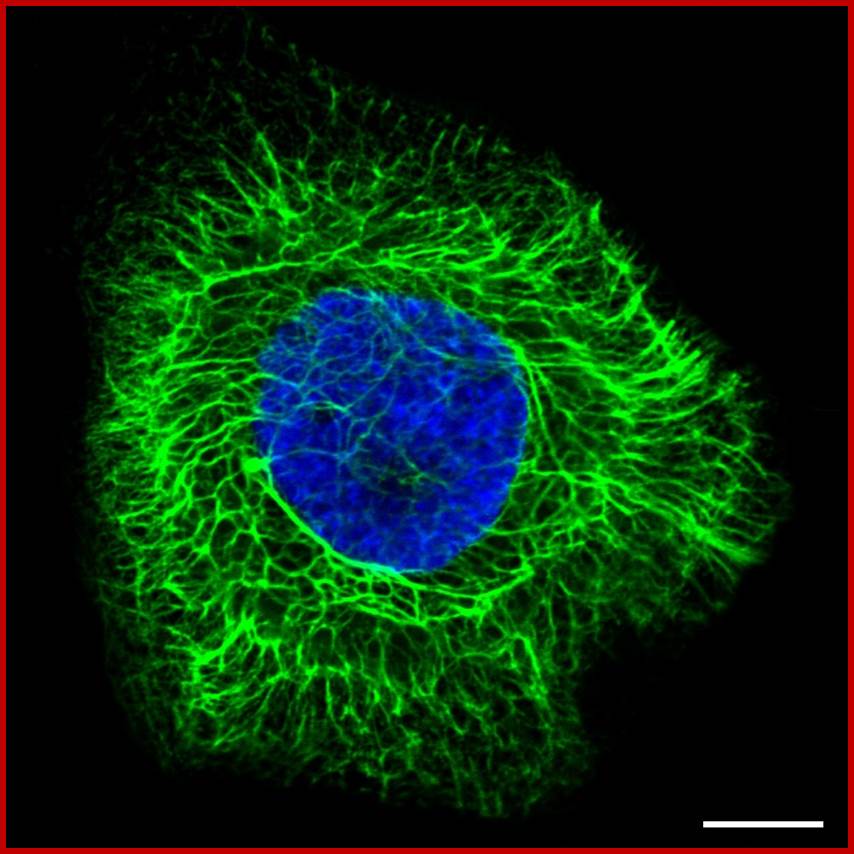
Cytoskeleton (intermediate filaments); http://www.proteinatlas.org/
Intermediate filaments have a twisted, rope-like structure that supports the cell structure. Cells that are subject to mechanical stress, such as hair and skin cells, often contain more intermediate filaments. Intermediate filaments are found both in the cytosol as well as attached to the inner side of the nuclear membrane. The cytosolic intermediate filaments are assigned to support and hold organelles in place. The nuclear laminas, which are attached to the inner nuclear membrane, support the membrane and provide anchorage sites for nuclear structures such as chromosomes and nuclear pores. Intermediate filaments are also involved in cell-cell contact, holding sheets of epithelial cells together via cell-cell (desmosome) junctions.
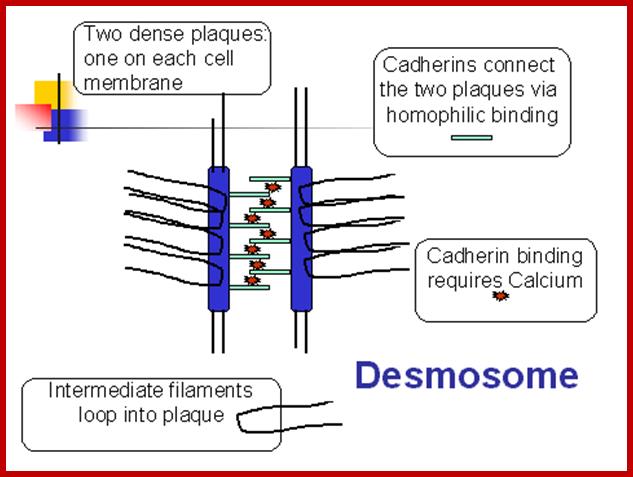
Two plaques on adjacent cells (containing desmoplakin and other proteins) are connected by cadherin molecules- they are called desmosomes; http://cytochemistry.net/
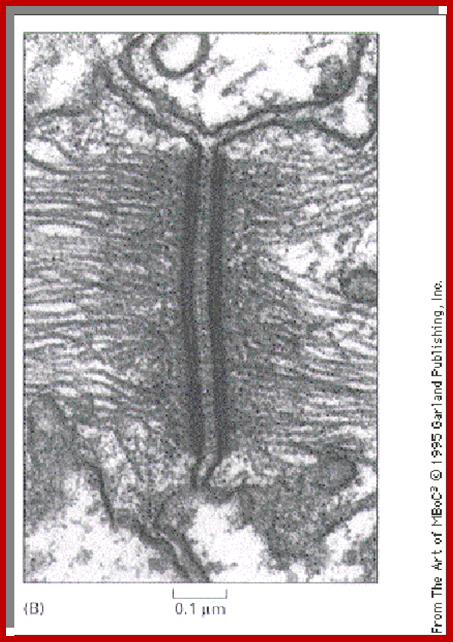
http://cytochemistry.net/
- The above cells are from skin and the cells look as if they have projected spines that touch spines from adjacent cells. Actually these are sites of desmosome connection which is a vital junction in the skin. The fixation technique has caused the cells to shrink, leaving the connection sites visible. An electron micrograph showing a desmosome is seen to the left. The intermediate filaments are looping in in almost a parallel fashion.
- Patients who make antibodies to cadherin molecules will have weak or absent desmosomes and the skin will form blisters. These fluid filled areas will lie in the regions where the cells with spines are found.
- Hemi desmosomes: Are sites of connections at the base of an epithelial cell with the matrix. The cartoon below shows the components. Intermediate filaments are stuck in a plaque (like the desmosome plaque) and Integrin molecules (receptors for matrix proteins) help connect the site with the matrix.
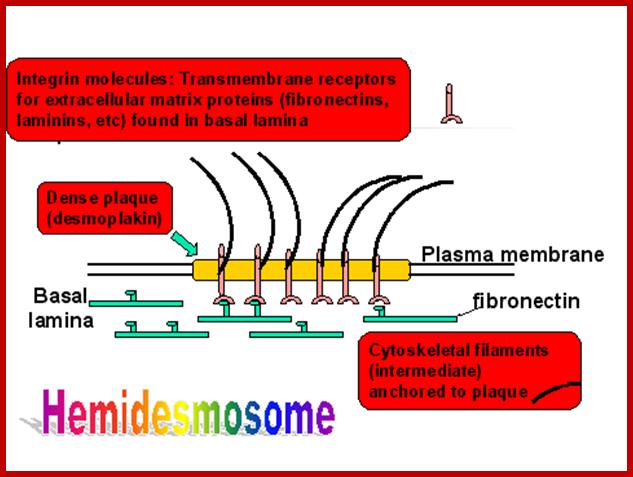 ‘
‘
http://cytochemistry.net/
- Type III Intermediate Filaments
- Found in a variety of cell types. Each is unique to that cell type and used to identify tissue containing that cell type. Vimentin is found in cells derived from mesoderm: fibroblasts, endothelial cells, white blood cells;
- Desmin is found in muscle cells, connecting Z disks and may connect center of contractile units. It is also found connecting to desmosomes in specialized junctions (cardiac muscle).
- Glial fibrillary acidic protein is found in glial cells in the central nervous system.
Type IV Intermediate filaments
- Include Neurofilament L, M, or H (named for low, medium or high molecular weight. These Neurofilament are linked by plectin cross bridges to each other and to microtubules. This adds to strength and spacing.
- Neurofilament proteins add to the diameter of the axon and therefore influence its function (larger axons conduct faster).