Plasma Membrane
No living cells on earth one finds without plasma membrane; it is one of the most important components of cells, which finds in prime position of the cell, whether it is a plant cell or an animal or bacterial cell or Archaeal cells.
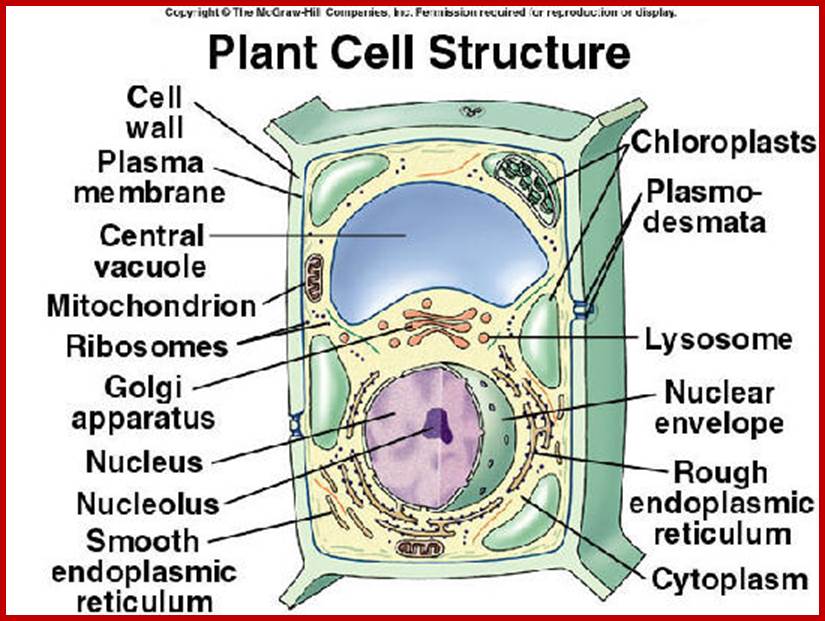
http://faculty.southwest.tn.edu/
Membranes are the most important structural components of the cell; it is the protecting layer of the cell bounding the protoplasm and provides the interface for interaction between the outer and inner components. All cells in all living systems have such membranes around the protoplasm. Various cell organelles too are bounded by membranes. Chemically, structurally cellular protoplasm is enveloped by a membrane called plasma membrane. It has very important functions such as receiving signals, involved in transport of chemicals inwards and outwards. In fact the active cytoplasmic fluid is pervaded with membranes. Some of the organelle membranes are highly specialized to perform specific functions. Most of the intracellular organelles are bounded by membranes, which actually make such structures compartmentalization of the protoplasm.
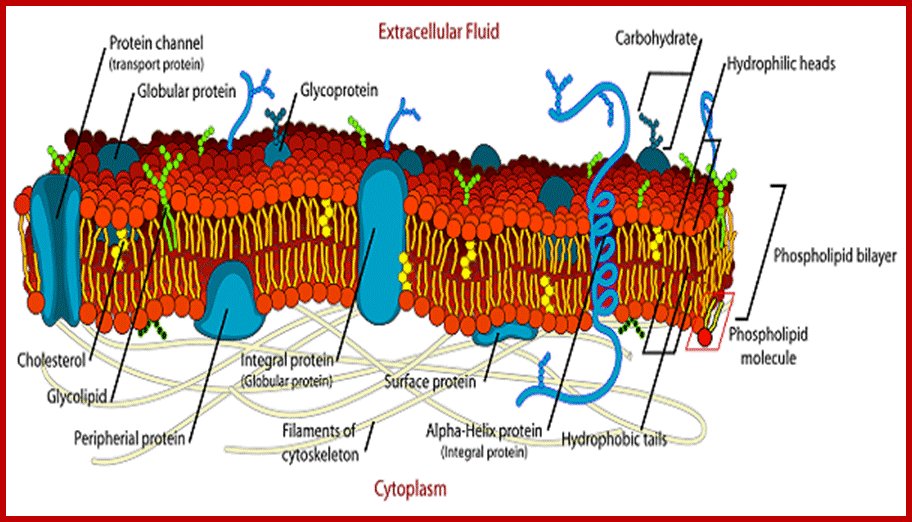
http://education-portal.com/; Phospholipid layers interspersed with proteins form a mosaic structure, but fluid and dynamic, which has different functions.
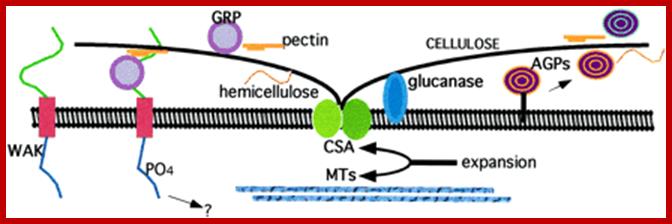
Plasma membrane- Cell Wall Contacts; http://www.plantphysiol.org/
Cellulose synthase (CSA) forms a rosette complex in the membrane and secrete cellulose. Underlying cellulose microfibrils have similar orientations. Ambinogabactus proteins (AGPs) are composed primarily of carbohydrates linked to smaller protein cores Some AGPs are anchored to membranes. Secreted AGPs and membrane bound forms are associated with a variety of cellular components perhaps provide adhesive or positional cues. Several glucanases are also bound to membranes. Several cell-wall associated kinases (WAK) having Ser/Thr kinase domains in the ECM (extracellular domains).
Cellulose is synthesized and secreted by CSA complex of cellulase synthase, between adjacent structures found in between adjacent cells. Pectin and hemicellulose are also synthesized and secreted through endomembranes and complexed with ECM as shown below. http://www.plantphysiol.org/
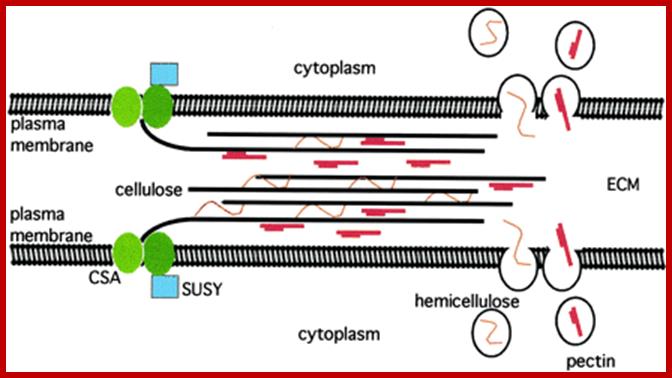
http://www.plantphysiol.org/
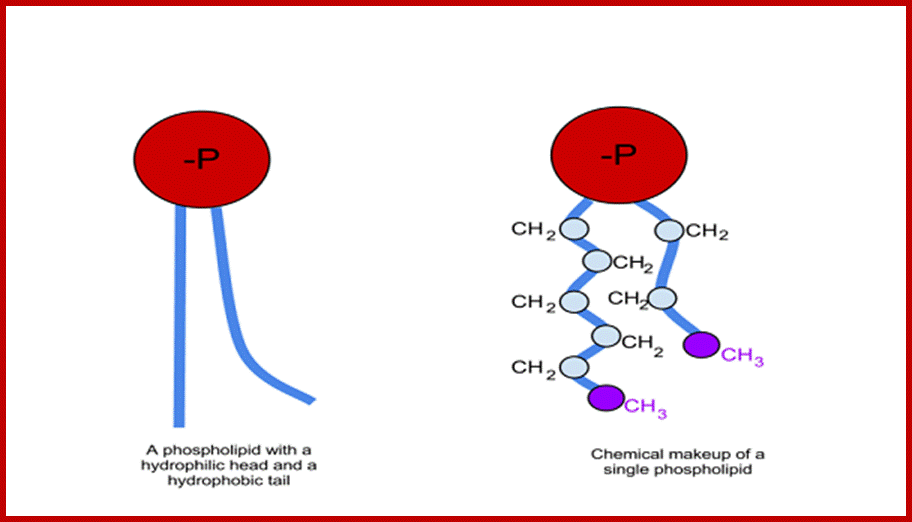
http://www.ias.ac.in/
Phospholipids are not the same in both layers, in plants they vary in their composition. Note all phospholipids have the same glycerol but the fatty acids attached can be different. Phosphoglycerol provide negative surface and fatty acids provide hydrophobic surface, hence fatty acid attracted to each other and form two layers.
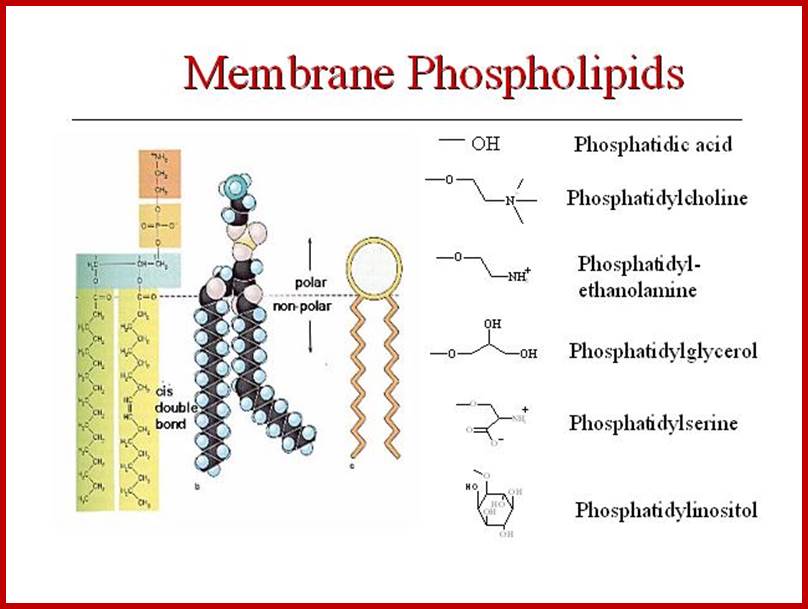
Phospholipid layer may contain cholesterol; Lipid to protein molar ration is ~50:1 to 100:1; the kind of phospholipids vary from one plant tissue to the other. In addition the composition of two layers cab vary; http://www.uic.edu/
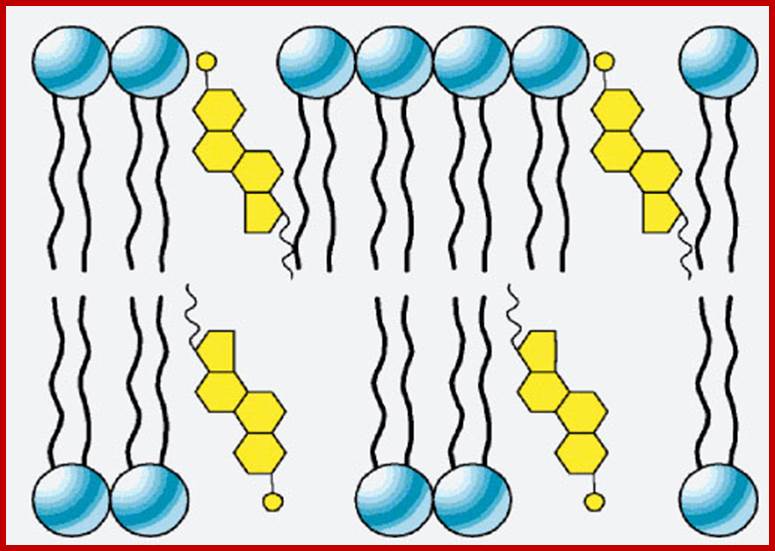
Asymmetric distribution of proteins and phospholipids are discerned by the diagram; http://en.wikibooks.org/
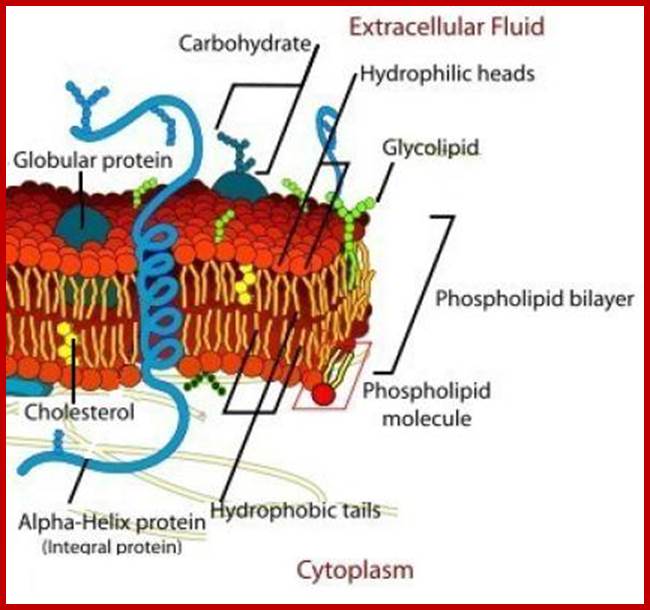
The cell membrane is a thin semi-permeable membrane that surrounds the cell's cytoplasm, enclosing its contents. Photo Credit: Image credit: Mariana Ruiz Villarreal; http://biology.about.com/
Extracellular and Intracellular Ion Concentrations of minerals;
|
Concentration (mM) |
||
|
Ion |
Intracellular |
Extracellular |
|
Squid axon |
||
|
K+ |
400 |
20 |
|
Na+ |
50 |
440 |
|
Cl- |
40–150 |
560 |
|
Ca2+ |
0.0001 |
10 |
|
Mammalian cell |
||
|
K+ |
140 |
5 |
|
Na+ |
5–15 |
145 |
|
Cl- |
4 |
110 |
|
Ca2+ |
0.0001 |
2.5–5 |
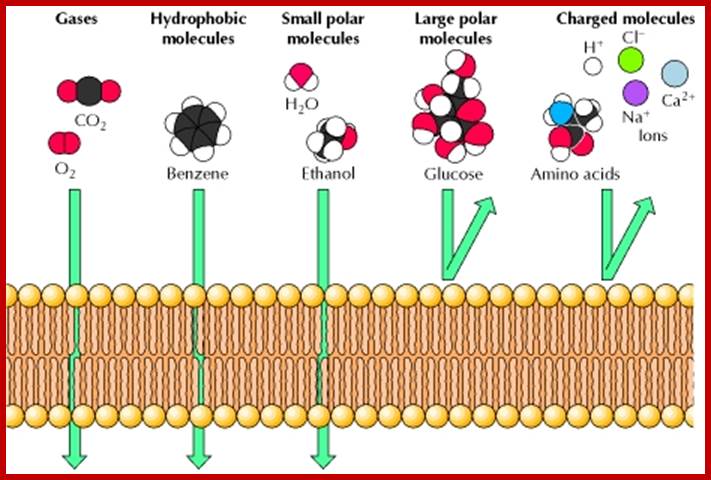
Permeability of phospholipid bilayers; Gases, hydrophobic molecules, and small polar uncharged molecules can diffuse through phospholipid bilayers. Larger polar molecules and charged molecules cannot diffuse across;. http://www.ncbi.nlm.nih.gov/
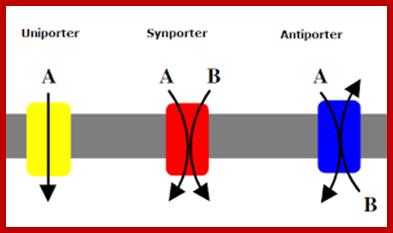
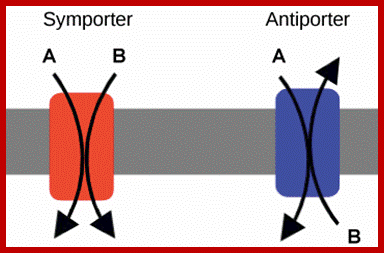
Co-transport in plants cell membranes; Na/H+ proton antiport through a pump; Co transport of Na/K+; Nitrate from soil /proton transport;
https://en.wikipedia.org; https://fr.khanacademy.org
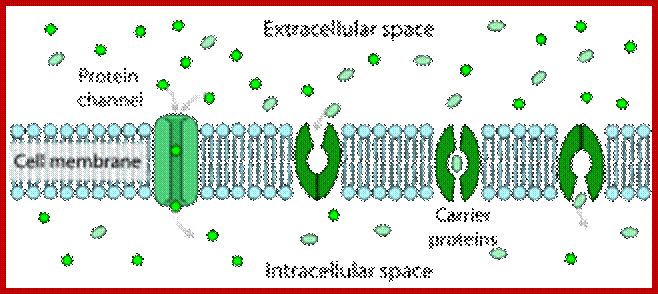
Facilitated diffusion in cell membranes showing ion channels and crrier proteins; https://en.wikipedia.org/wiki
Only the concentrations of Na+ and K+ are shown, because these are the ions that function in the transmission of nerve impulses. Na+ is pumped out of the cell while K+ is pumped in, so the concentration of Na+ is higher outside than inside of the axon, whereas the concentration of K+ is higher inside than out. The resting membrane is more permeable to K+ than to Na+ or other ions because it contains open K+ channels. The flow of K+ through these channels makes the major contribution to the resting membrane potential of -60 mV, which is therefore close to the K+ equilibrium potential. http://www.ncbi.nlm.nih.gov/
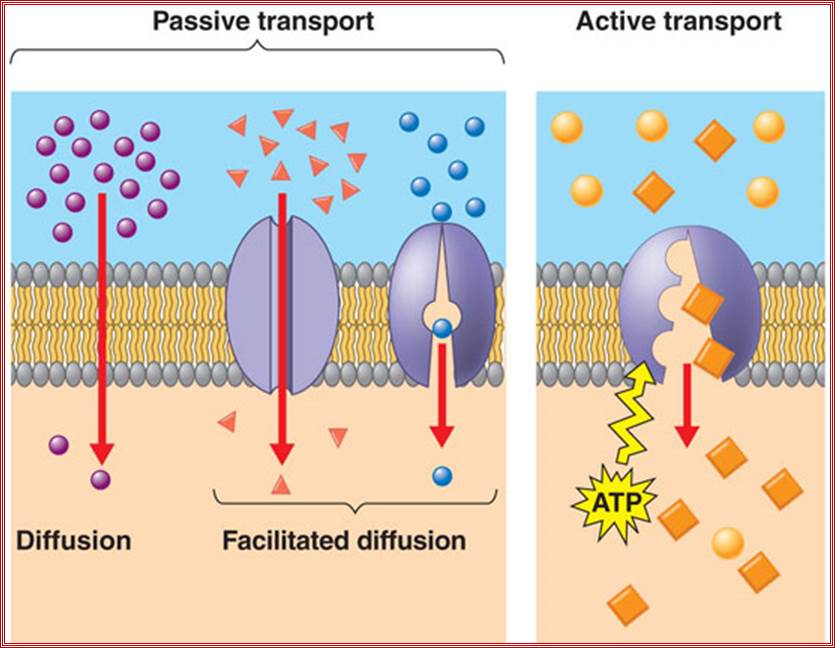
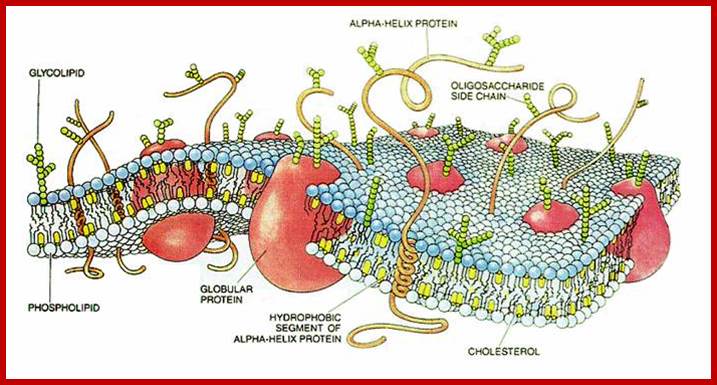
Lateral separation of lipids result in the formation of small domains called membrane rafts. These are rich in specific proteins, sterols and sphingolipids. Composition proteins are highly variable, some are found at the external surface and some at internal surface and some show transmembrane traverse the entire cross section. Surface proteins on the outer side are modified as glycoproteins. Many proteins act as receptors, and transmembrane transporters (both passive and active- or facilitated). The also contain Aquaporins for transport of water, which is also transported through diffusion. This is from PubMed. http://academygenbioii.pbworks.com/
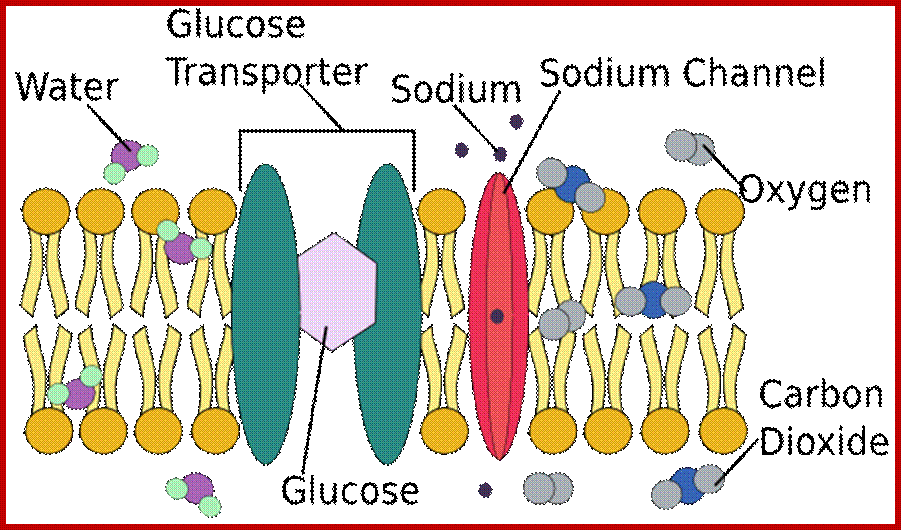
http://education-portal.com/
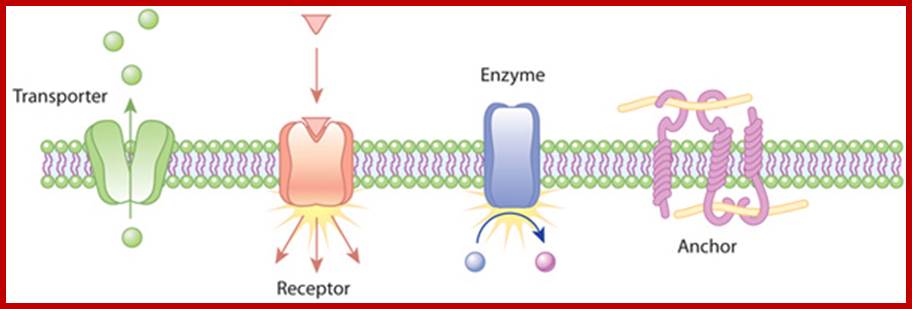
Role of transmembrane proteins; Transporters carry a molecule (such as glucose) from one side of the plasma membrane to the other. Receptors can bind an extracellular molecule (triangle), and this activates an intracellular processes. Enzymes in the membrane can do the same thing they do in the cytoplasm of a cell: transform a molecule into another form. Anchor proteins can physically link intracellular structures with extracellular structures. http://www.nature.com/
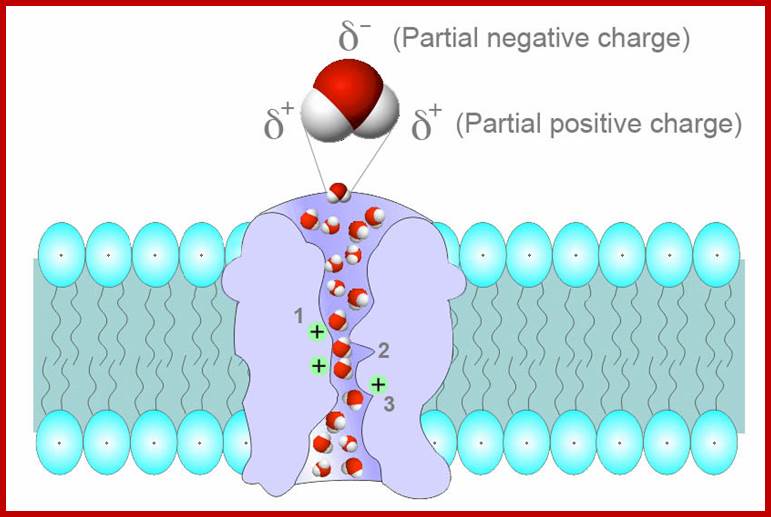
https://askabiologist.asu.edu;
Schematic depiction of water movement through the narrow selectivity filter of the aquaporin channel.; The 2003 Nobel Prize in Chemistry was awarded jointly to Peter Agre for the serendipity discovery of aquaporins 1993 and Roderick MacKinnon for his work on the structure and mechanism of potassium channels.;Aquaporins selectively conduct water in and out of the cells, they form a kind of water channels. Often they are also called glycroaquaporins; However the first report of protein mediated water transport through membranes was by Gheorghe Benga in 1986; in the membrane they are found and act as transmembrane tetrameric proteins; In plants they have a symplastic pathway for the movement of water; http://en.wikipedia.org/;
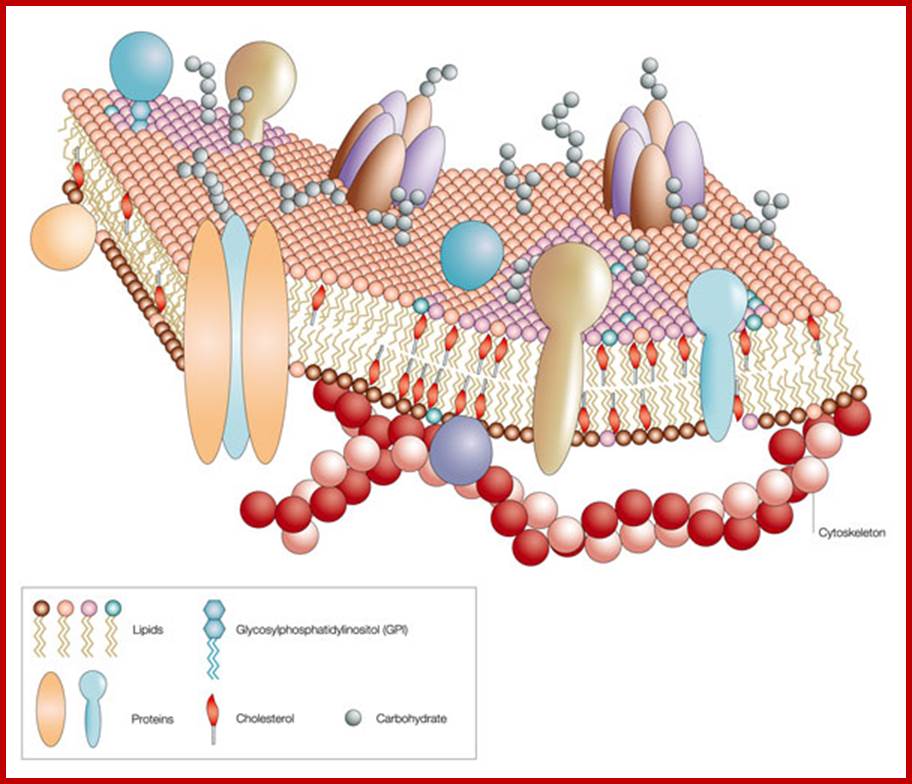
Like a mosaic, the cell membrane is a complex structure made up of many different parts, such as proteins, phospholipids, and cholesterol. The relative amounts of these components vary from membrane to membrane, and the types of lipids in membranes can also vary. http://www.nature.com/

Cell membranes and Transporters; www.study.com
Plant cell receptors:
Recent studies revealed that higher plants also possess genes coding for putative receptor kinases (Receptor-like Kinases, RLK). For instance, a completely sequenced Arabidopsis genome contains over 600 genes encoding RLKs (Shiu and Becker, 2001), suggesting that higher plants, like animals, use receptor kinase signaling commonly and broadly in responding to vast arrays of stimuli to modulate gene expressions. RLKs act as superfamily of receptors.
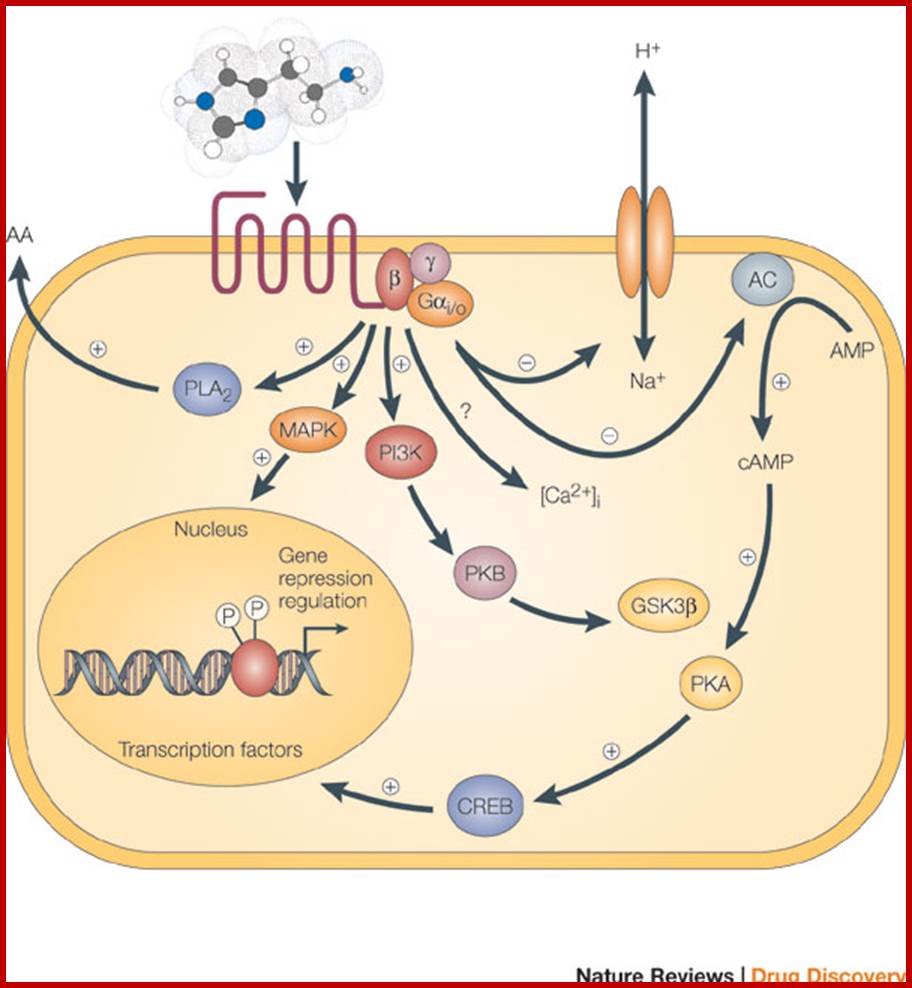
Binding of an agonist to the seven-transmembrane G-protein-coupled receptor in the plasma membrane activates a pathway that involves G proteins as well as cAMP-related pathways that modulate cellular signaling. In this example, the activated G alpha (Gαi/0) proteins inhibit (-) adenylyl cyclase (AC, on the right), the enzyme that induces formation of cAMP, which in turn results in the activation of protein kinase A (PKA). This in turn activates a molecule called cAMP-responsive element-binding protein (CREB), which modulates gene transcription. The activated G alpha proteins can also have a variety of other effects, shown at the left. These effects include activating the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways. Activation of the enzyme phospholipase A2 (PLA2) may also occur, which induces the release of arachidonic acid (AA), as well as inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ levels (exact mechanism unknown, ?). Subsequent activation of the MAPK and PI3K pathways results in the phosphorylation of extracellular signal-regulated kinases (ERKs) and protein kinase B (PKB), respectively. Activated PKB will subsequently phosphorylate and thereby inhibit the action of glycogen synthase kinase 3beta (GSK3beta), a major kinase in the brain. www.nature.com
© 2005 Nature Publishing Group Leurs, R. et al. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nature Reviews Drug Discovery 4, 107-120 (2005).
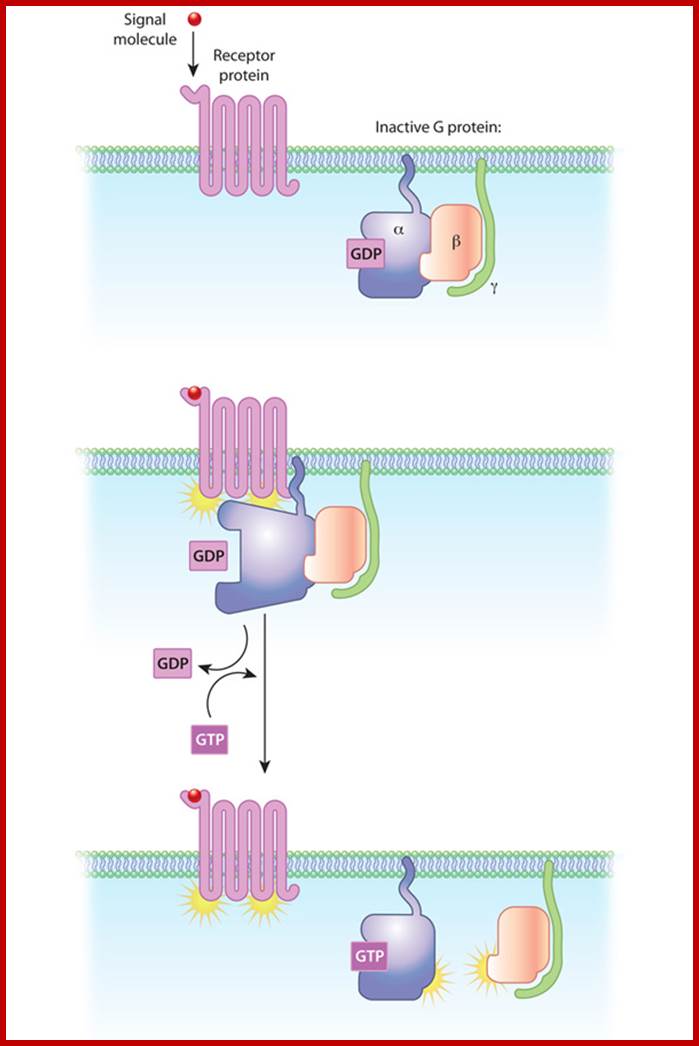
G proteins in Plasma membranes; In this diagram of G-protein-coupled receptor activation, the alpha, beta, and gamma subunits are shown with distinct relationships to the plasma membrane. After exchange of GDP with GTP on the alpha subunit, both the alpha subunit and the beta-gamma complex may interact with other molecules to promote signaling cascades. Note that both the alpha subunit and the beta-gamma complex remain tethered to the plasma membrane while they are activated. These activated subunits can act on ion channels in the cell membrane, as well as cellular enzymes and second messenger molecules that travel around the cell; http://www.nature.com/
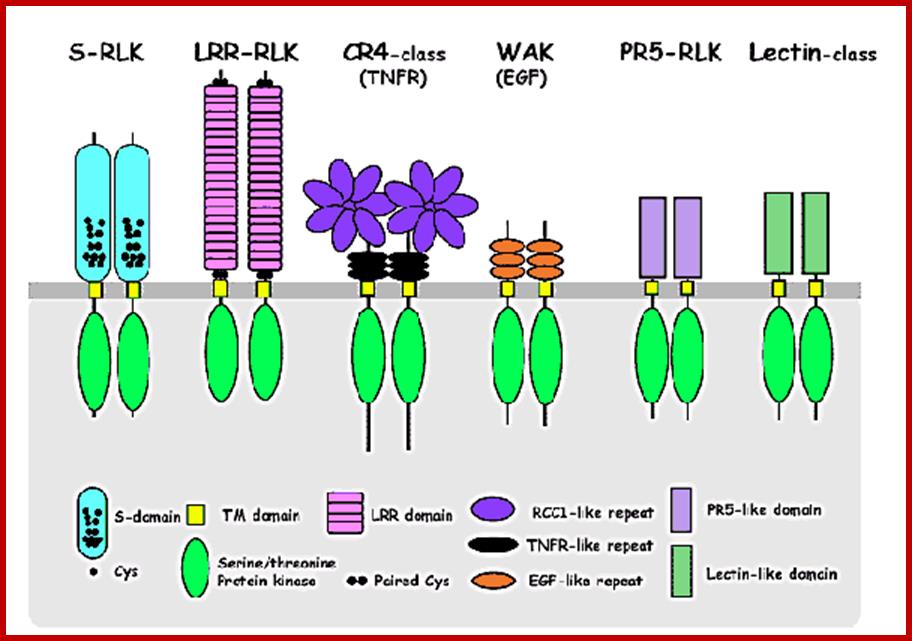
Plant RLKs are classified into subfamilies based on the structural feature of the extracellular domain, which is thought to act as a ligand-binding site. A common feature of these putative receptor kinases (RLKs), is that each has an N-terminal signal sequence, an extracellular domain that varies in structure, a single membrane-spanning region, and a cytoplasmic protein kinase catalytic domain. Unlike animals, where a majority of the receptor kinases possess tyrosine kinase activity, all of the plant RLKs thus far are shown to phosphorylate serine-and threonine residues, except one that displays dual specificity in vitro (Walker, 1994; Torii and Clark, 2000, many other great reviews are available!). S-domain class, TM domain class, LRR-domain class, RCCI-like repeats, PR5-Likedomain class, TNFR-like repeats, Lectin-like domains, Serine/threonine protein kinase class, EGF-like repeat class, PR-class; http://faculty.washington.edu/
Chemical Composition:
Analysis of chemical components found in membranes show variation from membrane-to-membrane types, from organelle to organelle. Generally, membranes are made up of proteins and lipids in various proportions as such as 1:1 to 1:3. The analysis of proteins and lipids of various types of membranes show wide variety of structural components.
Proteins are polymers of amino acid residues. They can be isolated and separated in the individual components by the methods of SDS, polyacrylamide gel electrophoresis, column chromatography and ammonium sulfate precipitation and other methods. Some of the membrane proteins are structural ones and others are found to be enzymes, receptors, transporters or carriers. Many of them are located at the outer face of the membranes, extrinsic proteins or they may be found in the core as intrinsic or integral proteins. Most of peripheral proteins are globular and they are either hydrophilic or partially hydrophobic. Integral proteins are however hydrophobic because they contain greater amount of nonpolar amino acids like leucine, Valine, isoleucine, etc at their surface. Even such proteins contain some hydrophilic amino acid residues. The presence of variety of proteins and protein complexes provide structural and functional heterogeneity to membranes.
Lipids:
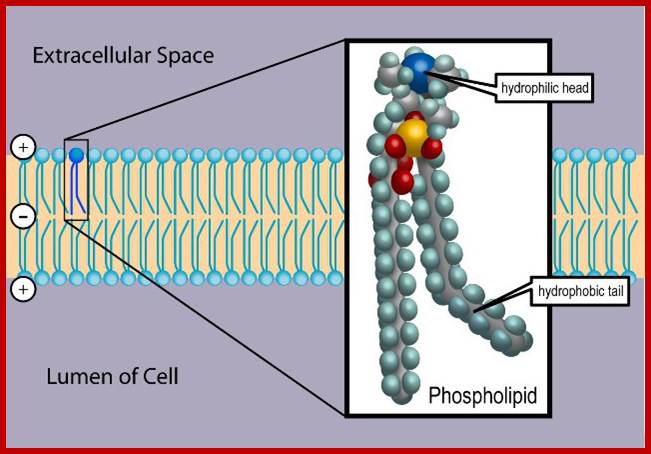
The only semi viscous to viscous part of the membranes contains a wide variety of lipids like phospholipids, sphingolipids, sulpholipids, phytosterols etc. Phospholipids are dipolar molecules with hydrophilic phosphate group at one and non polar hydrophobic fatty acid chains as tails at the other. Among them phosphotidyl serine, phosphotidyl ethanolamine phosphotidyl choline, phosphotidyl glycerol and cardiolipins are important.
Many of the lipids are associated with carbohydrates, such lipids are called glycolipids. Some of the membrane proteins are associated with carbohydrates and such proteins are called glycoproteins. They play important roles. Among sterols, cholesterols and phytosterols are common in plant membranes. Having polar heads and non polar tails, phospholipids play an important role in structural organization of the membrane.
Structure:
Starting from Sandwich model proposed by Danielli-Davson, the concept of membrane structure has undergone many modifications over the years. The unit membranes model of Robertson has been further improved by S.J. Singer and Garth L. Nicholson as Fluid Mosaic model. This model is the most accepted one today, for it explains most of the observed membrane structures and functions. Singer and Nicholson model is based on studies like Freeze fracture electron microscopy, Nuclear Magnetic Resonance, X-ray diffraction, Fluorescence spectroscopy and biochemical analytical techniques. This model has also taken into account of energy relations like translational movements, vibrational movements and hydrophobic, elctrostatic and hydrogen bond interactions. Moreover the dynamic feature of the membranes has been explained mostly on the basis of energy translations.
According to Fluid Mosaic Model, various phospholipids and other lipid components from a bilayered structure at the interface of water, because the hydrophobic tails of lipids get oriented towards each other in such a way the hydrophilic heads are exposed towards water. As wide variety of proteins of different dimension are integrated into lipid bilayers so as to form mosaic of lipids and proteins.
Many proteins are located at the interphase between water and hydrophilic phospholipid layers, some are held and buried in the hydrophobic core and other are integrated in the core of lipid bilayers so as to occupy the entire core section of the membrane. There is a dynamic interaction between lipids and proteins; they exhibit lateral movement including rotational flip flop turnovers. The position of proteins with respect to lipids in the membrane is never constant and always there is constant flux thus exhibit in quasi fluidity as well as quasi crystalline semi solid state. The association of microfilaments and microtubules at inner face of the membranes further adds up to its dynamicity to a greater extent. The above-described structure holds good for all the membranes. However, plasma membrane and other cytoplasmic membranes differ in their chemical composition particularly with respect to proteins and specific lipids. Even the thickness of the membrane varies from 60 – 100 Ĺ. The plasma lemma in most of the cells being the surface membrane contains a wide variety of receptor and carrier proteins. It produces invagination to produce cytosolic endoplasmic reticulum.
Functions:
Membranes being sheet like structures, posses a large surface area for many biochemical reactions. However, that function depends upon the protein and lipid contents. Plasma membrane as present at the outer surface, it has manifold function. Though they allow water to diffuse through in both directions, it prevents the free diffusion of both inorganic and organic solutes. Water movement is greatly facilitated by the presence of aquaporins. Thus, it exhibits semi permeable property. However, plasma lemma performs selective uptake of ions because specific solute carrier proteins found within the membrane. The plasma membrane is the first cellular structure that receives a wide variety of external stimuli like light, heat, chemicals, hormones, etc. Such stimuli are then passed on to cytoplasm or to the genetic material through specific receptor proteins. The surface membranes are also involved in bringing about changes in permeability and electro potential. They also take part in Pinocytosis and phagocytosis thus they facilitate the transportation of various substances in bulk. On the country, they are also responsible for the secretion of undigested materials and enzymes to the exterior surface. The presence of desmotubules of 200 A thicknesses, which traverse across the pit channels from one cell to another, is one of the unique features of the plasma membranes. Similar to plasma membrane other membranes also show specific functions. Thus, membranes exhibit dynamisity in its structure and function.
Biogenesis of Membranes:
Plasma membrane can be considered as an organ by itself; it exhibits a characteristic structure and functions. Plasma membranes of different cell types exhibit different functions either in receiving the stimulus or transportation of materials to and fro. In spite of its diversity and uniqueness the synthesis of plasma membrane mainly depends upon the activity of endoplasmic reticulum and Golgi complex. A large number of endoplasmic reticular membranes which are engaged in protein translocation and modifications contribute to the plasma membrane synthesis. Many of the fatty acids required are synthesized at the cytosolic side of the ER membranes for this region contains anchored enzymes. There is continuous flow of membrane materials from trans-golgi to plasma membranes and from plasma membrane inwards, there is dynamic equilibrium between these two components.
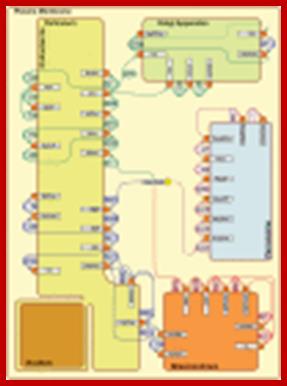
Compartmentalization of phospholipid biosynthetic activities; http://www.ncbi.nlm.nih.gov/
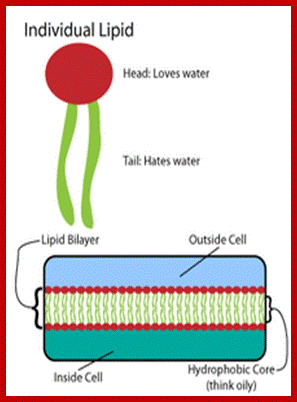
Lipid and Phospholipids mostly, individual, specific to specific membranes get incorporated; http://jonlieffmd.com
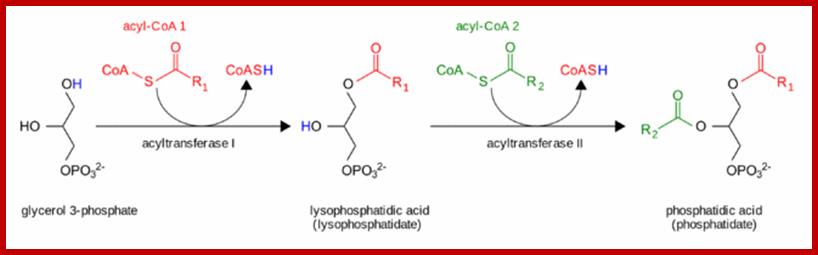
https://en.wikipedia.org
The pathway starts with glycerol 3-phosphate, which gets converted to lysophosphatidate via the addition of a fatty acid chain provided by acyl coenzyme A.[9] Then, lysophosphatidate is converted to phosphatides via the addition of another fatty acid chain contributed by a second acyl CoA; all of these steps are catalyzed by the glycerol phosphate acyltransferase enzyme.[9] Phospholipid synthesis continues in the endoplasmic reticulum, and the biosynthesis pathway diverges depending on the components of the particular phospholipid. https://en.wikipedia.org
GPAT, glycerol-3-phosphate O-acyltransferase; AGPAT, 1-acyl-sn-glycerol-3-phosphate O-acyltransferase; GPAT and AGPAT activities are associated with the ER and the mitochondria, providing the diacylglycerol phosphate (DGP) precursor for phospholipids in both locations. In the ER compartment, the DGP is dephosphorylated by the phosphatidic acid phosphatase enzymes to yield diacylglycerol (DG), which is incorporated into phosphatidylcholine (DGPCho) and phosphatidylethanolamine (DGPEtn). The GPAT and AGPAT association with the mitochondria suggests that these activities provide the DGP precursor for the synthesis of phosphatidylglycerol (DGPGro) and CL located at the same site. DGPCho is the most abundant glycerophospholipid species in mammalian cells, and it is synthesized in the ER and Golgi apparatus.
The ER and Golgi apparatus together constitute the endomembrane compartment in the cytoplasm of eukaryotic cells. The endomembrane compartment is a major site of lipid synthesis, and the ER is where not only lipids are synthesized, but membrane-bound proteins and secretory proteins are also made. ER and nuclear membranes form a continuous sheet enclosing a single internal space, called the lumen. the region in close proximity to the Golgi apparatus, rich in vesicles and tubules, is the ER-Golgi intermediate compartment (ERGIC). The ERGIC domain represents a continuum of the ER and Golgi apparatus where the lipids and lumenal proteins destined for transport to the cell surface or other organelles are transferred and biochemically modified. The cis-Golgi structure is in close proximity to the ERGIC, and the trans-Golgi network is the site for the formation of budding vesicles that distribute the lumenal protein contents. The ER interacts closely with the cytoskeleton, mostly with microtubules. This interaction allows the ER to maintain its position within the cell and facilitates intracellular trafficking, particularly from the smooth ER. Bulk membrane lipid biogenesis in primary cells largely occurs in the endomembrane compartment, which includes the domains of the ER and Golgi apparatus. Specialized phospholipids are synthesized in mitochondria or peroxisomes. Two proteins involved in the regulation of membrane phospholipid biogenesis are associated with the endomembrane compartment, namely, the CCT enzyme in the pathway for DGPCho, and the XBP-1(S) transcription factor. Activation of either protein occurs in response to changes in ER lipid or protein composition, respectively. ER membrane biogenesis can occur during developmental differentiation of secretory cells or in immune cells during the response to stimulation. De novo membrane phospholipid synthesis in differentiated cells is linked with secretion from the Golgi apparatus. The future challenge will be to sort out the relative importance of lipid synthesis or composition in the regulation of cellular events. Proteins synthesized in ER are assorted and transported selectively into PM and across PM http://www.ncbi.nlm.nih.gov/
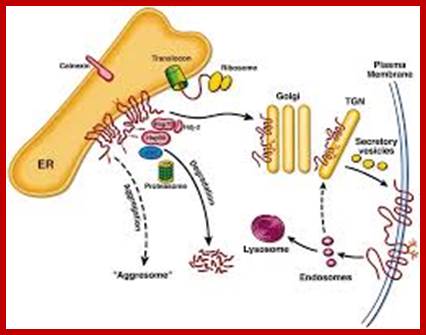
Fate of CFTR molecules synthesized on ER-associated ribosomes; As the primary structure is formed, the polypeptide is incorporated into the ER membrane. Core oligosaccharide chains are attached, to which calnexin binds. In addition, the cytosolic chaperones Hsp70, Hdj-2, and Hsp90 bind, and ubiquitination may occur. To be exported from the ER compartment in a productive manner via COPII-coated vesicles (not shown), a degree of higher structure must be achieved. This ATP-dependent conformational maturation is accompanied by dissociation of calnexin and the cytosolic chaperones. Fully folded CFTR is protected from degradation, but molecules that do not attain this conformation (»75% of wild-type molecules and »100% of DF508 molecules) are substrates of ubiquitinating enzymes (ubc) and are degraded by the proteasome. As-yet-unidentified proteases may also be involved, since degradation is not completely prevented by proteasome inhibition. When degradation is blocked or saturated, extensive aggregation of export-incompetent molecules occurs. The export-competent population travel from the ER through an ERGIC (not shown) to the Golgi apparatus, where complex oligosaccharide chains are completed. Vesicles then carry the completed molecule from the trans-Golgi network (TGN) to the plasma membrane. Endocytotic recycling of this population and degradation of some internalized protein by lysosomal proteases accounts for the turnover of surface-expressed CFTR.http://www.ncbi.nlm.nih.gov/http://jcs.biologists.org/