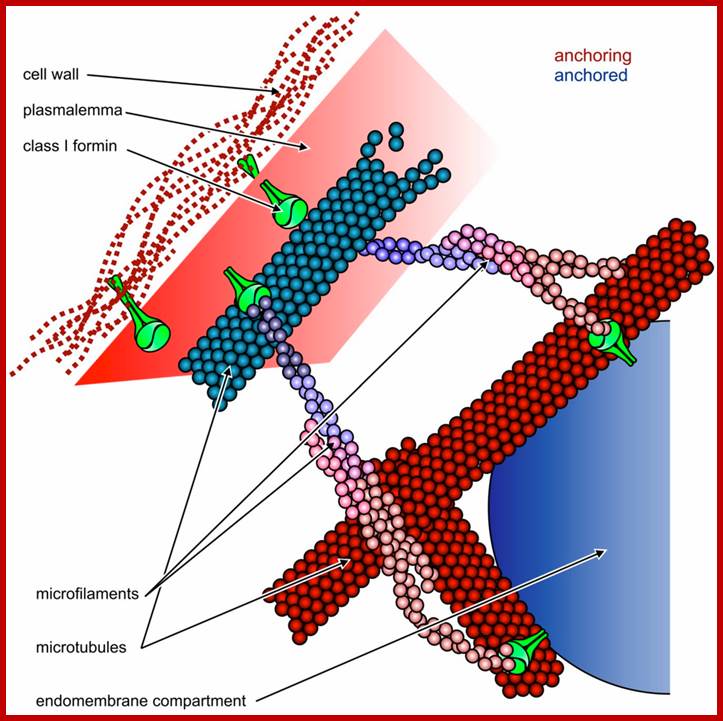
Endoplasmic Reticulum
Reticular network of membranes within cytoplasm is called endoplasmic reticulum (ER). The labyrinthine network of ER pervades the entire cytoplasmic fluid and provides continuous but a dynamic compartmentalization and fluidity. In fact plasma membrane and nuclear membrane are interconnected through various forms of endoplasmic reticulum. The ER is absent in RBC of mammals.
Endoplasmic Reticulum exhibits various forms and shapes; Flat cisternae 40-50Um thick, Vesicles 25-500um and Tubules 50-100um. Different cells show different patterns of ER membranes. The can easily change one for to the other. They also show variations in their chemical composition and structural organization. Some of the ER membranes are found as flat membranous sheets and some are in the form of branched tubular structures. They also exist as membranes vesicles. Nevertheless such forms are not constant and they undergo rapid changes from one from to another form and vice versa.
The endoplasmic reticulum (ER) is ramified, continuous membrane-bound organelle comprised of functionally and structurally distinct domains encompassing the nuclear envelope, peripheral tubular ER, peripheral cisternae, and numerous membrane contact sites at the plasma membrane, mitochondria, Golgi, endosomes, and peroxisomes. These are required for multiple functions, including synthesis of proteins and lipids, calcium level regulation, and exchange of macromolecules with various organelles at ER-membrane contact sites. The most important feature is protein synthesis and transport the same to golgi complex. The ER maintains its unique overall structure regardless of dynamics or transfer at ER-organelle contacts. In this review, we describe the numerous factors that contribute to the structure of the ER. http://cshperspectives.cshlp.org/
www.bp.blogspot.com/–iezQS1SNKo/Tka3zx8NG7I/AAAAAAAABMY/u4kyCri_suI/s1600/l-LTP4.png; http://www.yourarticlelibrary.com/

Density of ER in cytoplasm. Viewed through electron microscope http://micro.magnet.fsu.edu/

Rough ER for they are bond by translational ribosomes.www.doctorate.com

Rough and smooth ER; http://thecellorganelles.weebly.com/

http://thecellorganelles.weebly.com

The model for translational compartmentalization that has been generally accepted since the discovery of the signal recognition particle (SRP) is presented. Translation initiation begins in the cytosol. David W. Reid & Christopher V. Nicchitta; http://www.nature.com/

Flow of components from ER to Golgi vesicles, which is facilitated by COPI and COPII which show retrograde and anterograde movements EGTC.;http://jcs.biologists.org/
Though ER, is basically made up of two unit membranes enclosing a lumen, in between them, is filled with fluid. The extent and magnitude of ramification of this membrane system varies from cell type to cell type and stage of cell development. The cell that is actively engaged in protein synthesis and metabolically active contains greater amount of ER occupying more than 75% of the cytoplasmic space. ER membranes are distinguished into RER (rough ER) and SER (smooth ER) where RER is bound by ribosomes on its outer surface. In some animal systems the SER can be transformed into Sarcoplasmic reticulum SER, ex. RER components is more than SER.
In Myocytes sarcoplasmic reticulum stores Ca+2 ions and pumps the same into sarcoplasm, this happens when muscle are stimulated. Myocytes produce contractile fibers and deposit. Calcium ions interact with contractile fibers that utilize ATP to shorten the muscle fiber proteins. This SER (sarcoplasmic reticulum) plays an important role in excitation contraction coupling process.

Muscle fiber with sarcoplasmic reticulum in Blue; https://en.wikipedia.org
In RER, ribosomes’ the large subunit is bound to the membrane surface aided by specific receptors. The binding is attributed to the presence of two specific transmembrane proteins called ribophorins of mol wt. 65 KD and 63 KD respectively. These proteins are absent in SER. Note, SER and RER are not constant; RER can change to SER once ribosomes dissociate from ER after translation of mRNA is over. Most of the ribosomes found on RER membranes are in the form of polysome complexes actively engaged in protein synthesis. The nascent polypeptides thus synthesized enter into the lumen by its NH3 end through a groove found in the large ribosomal subunit. The first 20-40 amino acid recidues found at the NH2 end of the polypeptide chain act as signal polypeptides and they facilitate the translocation of proteins into the lumen of the RER. The proteins thus packed into RER lumen are transported towards SER to reach specific destinations. Some of the SER membranes carrying proteins mostly reach cis Golgi membranes or pinch off into vesicles which ultimately find their way into respective organelles. The ER membranes are supported by microtubular cytoskeleton structures. That is the reason they remain intact and also mobile. At the time of cell division at end of prophase MTs undergo depolymerization, with that ER collapse into fine vesicles. They reappear at the return of telophase, and MTs help in organizing the membranes.
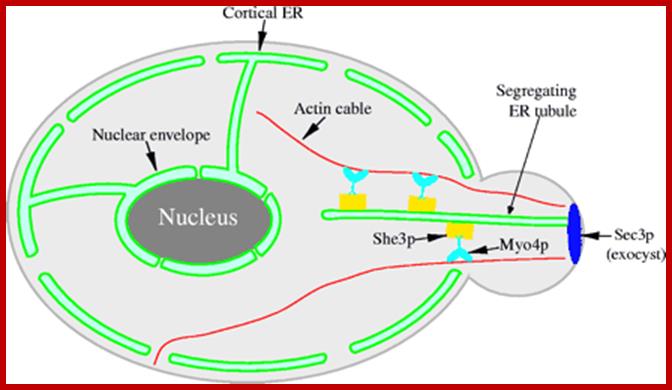


http://nayone-diary.blogspot.in/
Myo4p transports ER tubules into daughter cells along actin cables facilitated by motor proteins such as type V- myosin motor type of proteins; distribution of ER use cytoskeleton tracks and their motor proteins. The cytoskeleton is responsible for mediating these changes. By providing "tracks" with its protein filaments, the cytoskeleton allows organelles to move around within the cell. In addition to facilitating intracellular organelle movement, by moving itself the cytoskeleton can move the entire cells in multi-cellular organisms. In this way, the cytoskeleton is involved in intercellular communication. Organization of actin, microtubules, and intermediate filaments within a cell. http://jcs.biologists.org/; http://nayone-diary.blogspot.in/

Components of the microtubule-based axonal transport system. Stéphanie Millecamps & Jean-Pierre Julien; www.nature.com
ER tubules movement is supported by cytoskeleton elements like Microtubules. Actin acts as tracts for cortical ER movements. In cell axonal structure, mitochondria, golgi derived vesicles move to the ends where the axonal ends meet to cell synaptic surface; similarly many vesicles and mitochondria move to cell body facilitated by microtubules which act like tracks.
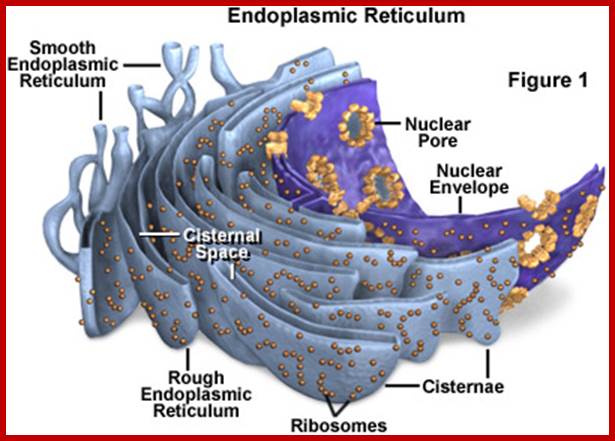
Possible roles of Class I plant formins such as AtFH8 (shown in green) in the organization of cytoskeletal and membrane structures. Relatively static structures shown in shades of red serve as a “dock” for anchoring more dynamic ones shown in blue. Top: formins can attach both microfilaments and microtubules to the plasmalemma, which is linked to the cell wall through integral membrane proteins, including the formins themselves (modified from [45]); Bottom: attachment of endomembrane compartments, such as the ER or secretory vesicles, to the cytoskeleton may be mediated by formins. While Class II formins cannot anchor the plasmalemma to the cell wall, they may also participate in endomembrane-cytoskeleton interactions as long as they bind to the membrane itself or to some integral or peripheral membrane proteins. http://www.mdpi.com/

Known microtubule-binding proteins on ER tubules and sheets.
(A) ER tubule. The mammalian ER tubule is shaped by proteins that stabilize tight membrane curvature. These proteins contain one or two hydrophobic hairpin segments that can embed into the cytoplasmic leaflet. The reticulons have no known microtubule-binding capacity but some members of the structurally similar REEPs (REEP1–4) have a carboxy-terminal microtubule-interacting region. Spastin is a microtubule-severing protein containing a hexameric AAA ATPase domain (the severing domain) and a separate microtubule-binding region. In addition, the spastin M1 variant has an amino-terminal extension containing one hairpin sequence that can bind ER. (B) ER sheet. Curvature-stabilizing proteins are excluded from the flat region of the sheet by mechanisms that are poorly understood. Among the sheet-enriched proteins are Climp63, p180 and kinectin. Climp63 has an extensive lumenal region that interacts homotypically and is thought to serve as the ‘spacer’ that maintains a 50 nm lumenal width. P180 has an extensive cytoplasmic domain that contains a microtubule-binding domain and may act in translation-independent localization of specific mRNAs to the ER membrane. Kinectin’s cytoplasmic region interacts with a region near the carboxyl terminus of kinesin. The diagrams are scaled to show relative diameters of ER (50 nm for both tubule and sheet) and microtubule (24 nm) and length of kinesin. The depictions of Climp63, p180 and kinectin show the approximate relative amounts within and without the ER lumen. ER membrane, green double line; proteins, orange; microtubule-binding region, yellow; kinesin-binding region, red; microtubule, blue. ;http://www.mdpi.com/.
https://online.science.psu.edu
Chemical Composition:
Endoplasmic reticular membranes contain high lipid content in relation to its protein partners. The common lipids found are phospholipids, phosphotidyl inositol, neutral lipids, sulfolipids, cholesterol and some phytosterols. Nearly 30-40 different types of ER membrane proteins have been isolated, of which many are enzymes like cytochrome P 450 and its subgroups, electron transport protein complexes like Cytochrome C reductase, Cytochrome b5 reductase. Gulcose 6 phosphotases are also common in ER. Furthermore, though ER shows similarity in their structural appearances they exhibit chemical heterogeneity at their cytoplasmic and lumen surfaces. They do contain a variety of resident proteins, which perform functions like, protein folding, protein modification and protein transport.
Biogenesis:
There are many views with regard to the biosynthesis of endomenbranes. According to one view, the membranes develop from plasma membranes by inward invagination and growth. But other view points out that ER develop from the outer nuclear membrane of the nucleus. The third view is ER develops by self-assembly. All the above said views point out that ER develops from both plasma membranes outer nuclear membrane and self-assembly. It is also true that both plasmamembranes and nuclear membrane are also derived from endomenbranes. It looks true of all those membrane bound organelles have some connection with ER. In protein synthesis and other cell membrane components are involved in the synthesis of various liquid components, thus they get self assembled by incorporating required proteins and lipid and other fatty acid derivatives.
Functions:
Endoplasmic membranes found in the cytoplasmic fluid are highly dynamic and show rapid but sweeping movements. In fact they exhibit high fluidity because the membranes are always in constant vesiculation and rejoining with other vesicles. Some of the important functions of RER is engaged in the synthesis of a wide variety of proteins at different sites and many of them are modified in terms glycosylation and adding of N-linked carbohydrate-complex. SER is involved in glycogenolysis. In plant cells SER is responsible for the secretion of vesicles containing raw materials for cell wall formation. SER is also responsible for the synthesis of various kinds of lipids and its derivatives such as phospholipids and sterols. ER enzymes like Cyt. P450 and its allied enzymes are capable of detoxification of drugs. Alcohols and other metabolically harmful components are detoxified; but the same can also cause damage to the liver.
The endoplasmic reticulum manufactures, processes, and transports a wide variety of biochemical compounds for use inside and outside of the cell. Consequently, many of the proteins found in the cisternal space of the endoplasmic reticulum lumen are there only transiently as they pass on their way to other locations. Other proteins, however, are targeted to constantly remain in the lumen and are known as endoplasmic reticulum resident proteins. These special proteins, which are necessary for the endoplasmic reticulum to carry out its normal functions, contain a specialized retention signal consisting of a specific sequence of amino acids that enables them to be retained by the organelle. An example of an important endoplasmic reticulum resident protein is the chaperone protein known as BiP (formally: the chaperone immunoglobulin-binding protein), which identifies other proteins that have been improperly built or processed and keeps them from being sent to their final destinations. http://micro.magnet.fsu.edu/

ER and mitochondrial associated reactive oxygen species (ROS) production under ER stress. ROS are generated in the ER as a part of an oxidative folding process during electron transfer between protein disulfide Isomerase (PDI) and endoplasmic reticulum oxidoreductin-1 (ERO-1). ER-induced oxidative stress is further tuned for the generation of mitochondrial ROS. Ca2+ ions released from the ER augments the production of mitochondrial ROS which induces the Kreb’s cycle to further induce oxidative phosphorylation at the electron transport chain (ETC). Moreover, Ca2+ ions increase cytochrome c release impairing electron transfer, altering mitochondrial membrane potential and increasing the generation of ROS. http://www.mdpi.com/

ER stress and unfolded protein response; http://ajpgi.physiology.org/
Endoplasmic reticulum (ER) stress is a phenomenon that occurs when excessive protein misfolding occurs during biosynthesis. ER stress triggers a series of signaling and transcriptional events known as the unfolded protein response (UPR). The UPR attempts to restore homeostasis in the ER but if unsuccessful can trigger apoptosis in the stressed cells and local inflammation. Intestinal secretory cells are susceptible to ER stress because they produce large amounts of complex proteins for secretion, most of which are involved in mucosal defense. This review focuses on ER stress in intestinal secretory cells and describes how increased protein misfolding could occur in these cells, the process of degradation of misfolded proteins, the major molecular elements of the UPR pathway, and links between the UPR and inflammation. Evidence is reviewed from mouse models and human inflammatory bowel diseases that ties ER stress and activation of the UPR with intestinal inflammation, and possible therapeutic approaches to ameliorate ER stress are discussed. The smooth endoplasmic reticulum in most cells is much less extensive than the rough endoplasmic reticulum and is sometimes alternatively termed transitional. Smooth endoplasmic reticulum is chiefly involved, however, with the production of lipids (fats), building blocks for carbohydrate metabolism, and the detoxification of drugs and poisons. Therefore, in some specialized cells, such as those that are occupied chiefly in lipid and carbohydrate metabolism (brain and muscle) or detoxification (liver), the smooth endoplasmic reticulum is much more extensive and is crucial to cellular function. Smooth endoplasmic reticulum also plays a role in various cellular activities through its storage of calcium and involvement in calcium metabolism. In muscle cells, smooth endoplasmic reticulum releases calcium to trigger muscle contractions. Presented in Figure 2 is a fluorescence digital image taken through the microscope of the endoplasmic reticulum network in a bovine (cow) pulmonary artery endothelial cell grown in culture.

Unfolded proteins are transported out of ER into cytosol where they are degraded.in ubiquitin pathway

https://en.wikipedia.org

Proteins (bad) transported back into cytosol for degradation; http://www.scs.illinois.edu/

Retrograde transport from Golgi to ER: www.studyblue.com

http://www.slideshare.net/

Certain proteins from cis golgi are retrived back to ER via signal recognition sequences; http://quizlet.com/
1. Luminal proteins: Proteins found in the lumen of the Golgi complex that need to be transported to the lumen of the ER contain the signal peptide KDEL. This sequence is recognized by a membrane-bound KDEL receptor. In yeast, this is Erd2p and in mammals it is KDELR. This receptor then binds to an ARF-GEF, a class of guanine nucleotide exchange factors. This protein in turn binds to the ARF. This interaction causes ARF to exchange its bound GDP for GTP. Once this exchange is made ARF binds to the cytosolic side of the cis-Golgi membrane.
2. Membrane proteins: Transmembrane proteins which reside in the ER contain sorting signals in their cytosolic tails which direct the protein to exit the Golgi and return to the ER. These sorting signals, or motifs, typically contain the amino acid sequence KKXX, which interact with COPI. The order in which adaptor proteins associate with cargo, or adaptor proteins associate with ARFs is unclear, however, in order to form a mature transport carrier coat protein, adaptor, cargo, and ARF must all associate.

Network of ER; www.biology.arizona.edu

Ribosomes bound to ER; http://jennarever.weebly.com/

Transition from RER to SER; http://cronodon.com/BioTech
ER with MAMs:

ER with Mitochondrial Membranes (MAMs)

Structure and functions of mitochondria. Mitochondrial membranes and compartments comprise the matrix, inner mitochondrial membrane (IMM), outer mitochondrial membrane (OMM), cristae and mitochondria-associated membrane (MAM). MAMs are points of contact between mitochondria and smooth endoplasmic reticulum (ER), enriched in certain factors, including the inositol triphosphate receptor (IP3R), voltage-dependent anion channel (VDAC) and sarco/endoplasmic reticulum calcium ATPase (SERCA). The main functions of mitochondria are represented in color codes. In red: fatty acids and acetyl coenzyme A (acetyl-CoA) are oxidized by β-oxidation (also called Lynen helix) and the tricarboxylic acid cycle (TCA), respectively, and energy is transferred onto redox coenzymes. In green: the electron transport chain (ETC) is composed of five respiratory complexes (numbered I to V) and uses the electrons of the coenzymes generated by β-oxidation and the TCA cycle as substrates to generate a proton gradient (mtΔΨ) across the inner membrane. The flux of protons from the intermembrane space through complex V (ATP synthase) back into the mitochondrial matrix finally leads to ATP generation. ATP is exported to cytosol via adenine nucleotide translocase (ANT). In brown: electrons that leak from the ETC react with O2, forming superoxide anion O2•- and hydrogen peroxide H2O2, also referred to as reactive oxygen species (ROS). In blue: a number of caspase activators are retained in the intermitochondrial membrane space, including, e.g., cytochrome c, endonuclease G and apoptosis-inducing factor (AIF). These are released into the cytosol by pro-apoptotic signaling, which leads to opening of the mitochondrial permeability transition pore (mPTP). In violet: mitochondria constantly exchange calcium with the cytosol via the mitochondrial calcium uniporter (MCU) and Na+Ca2+ exchanger (NaCaE) and with the ER via the mPTP, which comprises, e.g., IP3R and VDAC. http://www.mdpi.com/


Chronichepatites C causes metabolic disorders lead to cancer decvelopement. Many lines of evidence suggest that mitochondrial dysfunctions, including modification of metabolic fluxes, generation and elimination of oxidative stress, Ca2+ signaling and apoptosis, play a central role in these processes. However, how these dysfunctions are induced by the virus and whether they play a role in disease progression and neoplastic transformation remains to be determined. Most in vitrostudies performed so far have shown that several of the hepatitis C virus (HCV) proteins localize to mitochondria, but the consequences of these interactions on mitochondrial functions remain contradictory, probably due to the use of artificial expression and replication systems.The ER is the major site ofr storage of Ca2+.Alteration in oxidative environmenrt in ERand also intra ER Ca2+ results ER stress induced reactive Oxygen (RoS); www.mdpi.com

Hypothetical models of the role of contacts between mitochondria and ER in apoptosis. The hFis1/Bap31 platform transmits the mitochondrial stress signal to the ER via the activation of procaspase-8. The cytosolic region of the ER integral membrane protein Bap31 is cleaved by activated caspase-8 to generate proapoptotic p20Bap31, which causes rapid transmission of ER calcium signals to the mitochondria via the IP3 receptor. At close ER-mitochondria contact sites, mitochondria takes up calcium into the matrix via the mitochondrial calcium channels MICU1 or LETM1. The massive influx of calcium leads to mitochondrial fission, cristae remodeling, and cytochrome release. Mfn2 is enriched in the mitochondria-associated membranes (MAM) of the endoplasmic reticulum (ER), where it interacts with Mfn1 and Mfn2 on the mitochondria to form interorganellar bridges. Upon apoptosis signal, a BH3-only member of the Bcl-2 family, Bik, induces Ca2+ release from the ER and, in turn, induces Drp1 recruitment to the mitochondria and their fragmentation and cristae remodeling. SERCA, sarco/endoplasmic reticulum Ca2+-ATPase. MICU1, mitochondrial calcium uptake 1. LETM1, leucine zipper/EF hand-containing transmembrane 1; http://www.hindawi.com/

Mitochondria are cellular organelles involved in host‐cell metabolic processes and the control of programmed cell death. A direct link between mitochondria and innate immune signalling was first highlighted with the identification of MAVS—a crucial adaptor for RIGI‐like receptor signalling—as a mitochondria‐anchored protein. Recently, other innate immune molecules, such as NLRX1, TRAF6, NLRP3 and IRGM have been functionally associated with mitochondria. Furthermore, mitochondrial alarmins—such as mitochondrial DNA and formyl peptides—can be released by damaged mitochondria and trigger inflammation. Therefore, mitochondria emerge as a fundamental hub for innate immune signalling. http://embor.embopress.org/
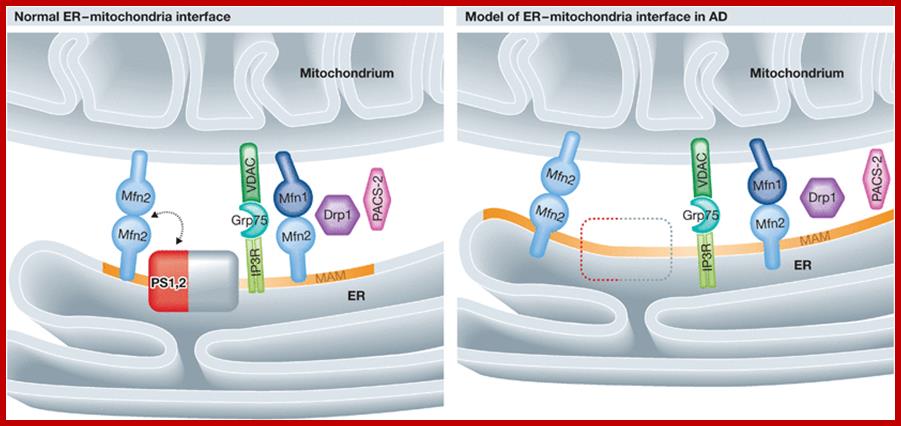
Close encounter: mitochondria, endoplasmic reticulum and Alzheimer's disease; Alzheimer's disease (AD) is characterized by the loss of hippocampal and cortical neurons as a consequence of the accumulation of amyloid‐β (Aβ). Aβ is produced from the amyloid precursor protein (APP) by the γ‐secretase complex components presenilin‐1 (PS1) and ‐2 (PS2), which are mutated in genetic forms of AD. In this issue, Schon and coworkers show that PS1 and PS2 are located at the interface between mitochondria and endoplasmic reticulum (ER). In models of familial and sporadic AD, these two organelles are juxtaposed closely, affecting shared lipid metabolic pathways. The interface between mitochondria and ER emerges as a new potential determinant of AD pathogenesis. Mfn, mitofusin; Drp1, dynamin‐related protein 1; IP3R, inositol triphosphate receptor; VDAC, voltage‐dependent anion channel; GRP75, heat‐shock protein 75; PS, presenilin; the orange region on the ER illustrates the MAM. Bart De Strooper Luca Scorrano; http://emboj.embopress.org/