Golgi Bodies:
Camillo Golgi discovered certain peculiar ultramicroscopic membranous structures in animal cells which showed complex organization. And there were named after him as Golgi complex or Golgi bodies or Golgi membranes. But biologists call such structures found in plant cells as dictyosomes.
Golgi bodies look like a stack of flat membranous sacs of various sizes and dimensions and form a complex. The organelles measure 2 mm to 5 mm in size and consists of 6-20 double membranes stacked one above the other or side by side. They are found in large numbers particularly in secretory tissues like salivary glands, stigmatic surface cells, tapetal cells and glandular tissues of Drosera and other insectivorous plants. But other cells contain 2 -5 Golgi bodies.
Structure and Biogenesis:
Golgi bodies rather call it as Golgi complex is a specialized membranes designed to perform some specific functions. They are made up of a stack of flat circular or perforated membranous sacs containing lumen of 100 to 200 Å thicknesses. The number of membranous sacs vary from 10 to 20 with an inter spaces of 200 to 300 Å.
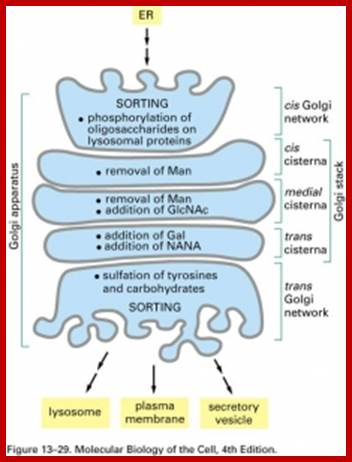
Such membrane complexes are associated with SER membranes at the formation face which is slightly convex in its topology called cis Golgi face. The other face is called maturation face called Tran Golgi face. At the formation face smaller membranous sacs are is association with numerous vesicles derived from SER. In fact one can observe a transition from RER to SER and from SER to Golgi vesicles at the formation face. The vesicles thus derived from various SER membranes fuse with one another. So one finds the proteins that are synthesized on RER ultimately find their way into Golgi membranes at the proximal face. Even the lipids that are synthesized in SER and mono or disaccharides accumulated in SER find their way into Golgi membranes. Whole lot proteins and enzymes are loaded in ER lumen. Formation of vesicles from SER and fusion of them into a flat sac is a continuous process, thus many such sacs are stacked one above the other. However, various components like proteins, lipids and carbohydrates are further processed and packed at the peripheral region of membranous sacs which develop into enlarged bulbous structures. Once such packaging process is completed, the membranes pinch off vesicles and the membranes disappear at the maturation face. Thus one can observe various stages of processing and packaging of different components loaded into vesicles in the complex. The membrane flow from SER to Golgi, Golgi to SER retrograde movement and the forward movement is called anterograde movement. Movement from trans Golgi to other region is performed by a variety of transport proteins such as COPs Coat Proteins, Clathrins and Adaptor and adaptin proteins.
Time lapse microphotography clearly shows that the total time required from the assembly of vesicles at the formation face and release of vesicles at the maturation face, is about 20-40 minutes. The formation of Golgi membranes at one face and disappearance of such membranes at the other is a continuous process, but the activity of golgi complex is very high in certain specialized tissues where they are involved in secretion.
Chemical Composition:
Techniques like equilibrium density gradient centrifugation have come handy in isolating fairly pure Golgi membranes. The chemical analysis of such membrane components reveal that the Golgi membranes are made up of 60 per cent proteins and 40 per cent lipids. Some of the proteins found in Golgi bodies are same as that of endoplasmic reticulum. Nonetheless they differ from ER in other protein components, because the processed and packed proteins vary. But with respect to lipid components, particularly in plant Golgi membranes they have more of phosphotidic acids and phosphotidyl glycerol. Such Golgi membranes are specifically lacking in sialic acids which are predominant components in animal liver Golgi membranes. Plant Golgi membranes also contain carbohydrates like glucosamines, galactose, glucose, mannose, fucose, xylose, arabinose and other sugars. Furthermore NAD dependent enzymes, such as Cyt.C oxidase and Cyt B reductases are found in the membranes. C oxidase, NAD dependent enzymes and Cyt.B are found in Golgi membranes. The most characteristic rather marked enzymatic components are glycosyl transferase and thiamine pyrophosphotase. Acid phosphotase enzymes are also found because primary lysosomal structures are still in the process of formation and release.
Functions:
Various cellular components are loaded into SER, then transported and processed packed into Golgi membranes before they are released into cytosol as vesicles. The problem that still puzzles biologists is that, how different components are sorted out and pack them into asserted groups. One of the possible explanations is that as the incoming products are synthesized on a particular set of polysomal complex on RER the proteins should be same. Then they are processed and packed into a single vesicle. Different components, coming from different SER membranes in from different directions, are separate into different groups at the formation face of the Golgi complex itself; later these are processed and packed into different vesicles. The golgi vesicles do contain receptors for specific epitopes, thus each and every transit proteins are recognized and sorted out.
In plant cells, Golgi bodies process and pack enzymes and carbohydrates into the same vesicles or different vesicles for the synthesis of cell walls. Later such vesicles are transported towards plasma membranes, microtubules direct the transport. Lysosomal enzymes are also packed into different vesicles. Some of the components of micro bodies or glyoxysomes or peroxisomes, mitochondria, nucleus and plastids receive proteins from cytoplasm directly. It is also known that some of the mucilaginous products in plants are produced by Golgi complex.
Golgi membranes are also involved in the aggregation of many enzyme precursors into zymogen granules which get enclosed in the vesicles. Later they are transported to different regions where the precursor proteins are activated. But proteins like insulin in animal cells are processed into active molecules and packed into vesicles which are then released into outer spaces.
Furthermore the membranes of Golgi complexes are involved in lipid accumulation. Such lipid components are either glycosylated or and proteinated to produce glycolipids and lipoproteins respectively. In animal cells, during spermatid maturation, the required acrosomal components are produced by Golgi membranes. They are also responsible for packing a group of hydrolyzing enzymes into primary lysosomal structures.
An important feature of processing, sorting and packing of different components into different vesicles is the requirement of metabolic energy like ATP. Thus Golgi membranes perform functions like receiving, sorting, modifying, packaging and releasing various components into their individual functional but targeted vesicles, which ultimately reach specific destinations to perform their functions. Remarkable organelle indeed!
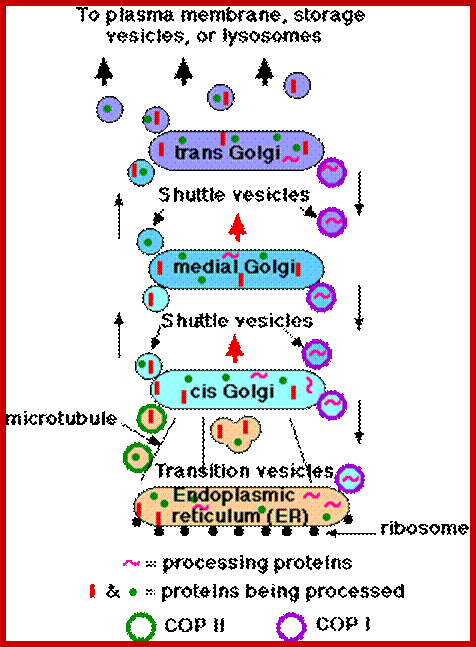
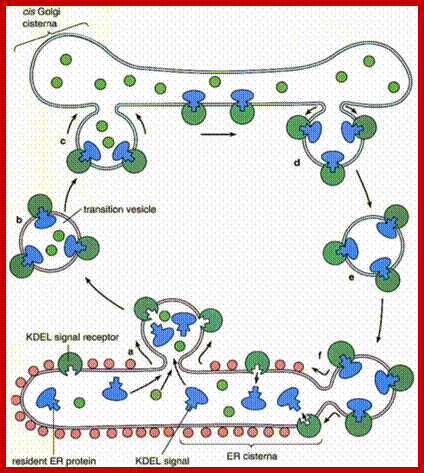
Inbound path: The movement of cisternal contents through the stack means that essential processing enzymes are also moving away from their proper site of action. Using a variety of signals, the Golgi separates the products from the processing enzymes that made them and returns the enzymes back to the endoplasmic reticulum. This transport is also done by pinching off vesicles, but the inbound vesicles are coated with COPI (coat protein I), http://users.rcn.com/
Out bound Path:
- Transition vesicles pinch off from the surface of the endoplasmic reticulum carrying
- integral membrane proteins
- soluble proteins awaiting processing
- processing enzymes
- Pinching off requires that the vesicle be coated with COPII (Coat Protein II)
- The transition vesicles move toward the cis Golgi on microtubules.
- As they do so, their COPII coat is removed and they may fuse together forming larger vesicles.
- These fuse with the cis Golgi
- Sugars are added to proteins in small packets so many glycoproteins have to undergo a large number of sequential steps of glycosylation, each requiring its own enzymes.
- These steps take place as shuttle vesicles carry the proteins from cis to medial to the trans Golgi compartments.
- At the outer face of the trans Golgi, vesicles pinch off and carry their completed products to their various destinations.
How does a vesicle recognize its correct target?
 This involves pairs of complementary integral membrane
proteins.
This involves pairs of complementary integral membrane
proteins.
- v-SNAREs = "vesicle SNAREs" — on the vesicle surface;
- t-SNAREs = "target SNAREs" — on the surface of the target membrane.
v-SNAREs and t-SNAREs bind specifically to each other thanks to the complementary structure of their surface domains.
Binding is followed by fusion of the two membranes.
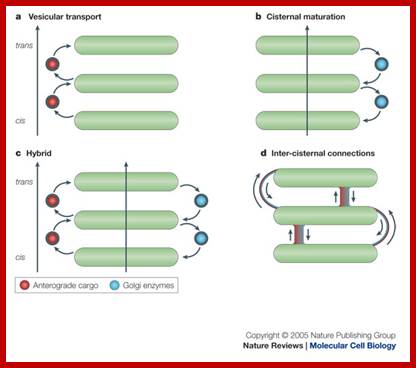
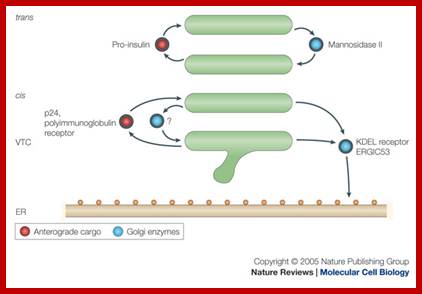
An overview of proposed roles of COP1 vesicles; Catherine Raboulle et al: www.nature.com
The upper figure: In the vesicular transport, Coatomer protein complex vesicles carry cargo and move in anterograde fashion from one golgi cisternae to the next, b. In the cisternal maturation model, COP1 vesicles move in retrograde fashion and function as retrieving device that is used by golgi enzymes to maintain their specific and differential localization over the Golgi sac. The hybrid model proposes that COPI vesicles mediate both anterograde and retrograde movement of cargo. Inter cisternal connections model does not involve COPI vesicles and proposes that cargo-resident enzymes move forwards and backwards, respectively through tubules that connect the rims and the core of heterologous cisternae in a given Golgi sac
Lower figure: Coatomer complex COPI mediate retrograde movement of the KDEL (a receptor binds the ER-retention sequence KDEL) and ERGIC 53 (ER-Golgi intermediate compartment 53kDa protein) from the cis golgi cisternae and VTC (vesicular-tubular clusters) back to ER; the retrograde movement of golgi enzymes (Mannosidase II) with golgi stack; the anterograde movement of cargo proteins (ex. Pro-insulin, 24, and polyimmunoglobulin receptor); and possibly the retrograde movement of proteins from the cis-Golgi cisternae to VTC trans, trans golgi cisterna; catherine Rabouille and Judith Klumperman; www.Nature.com
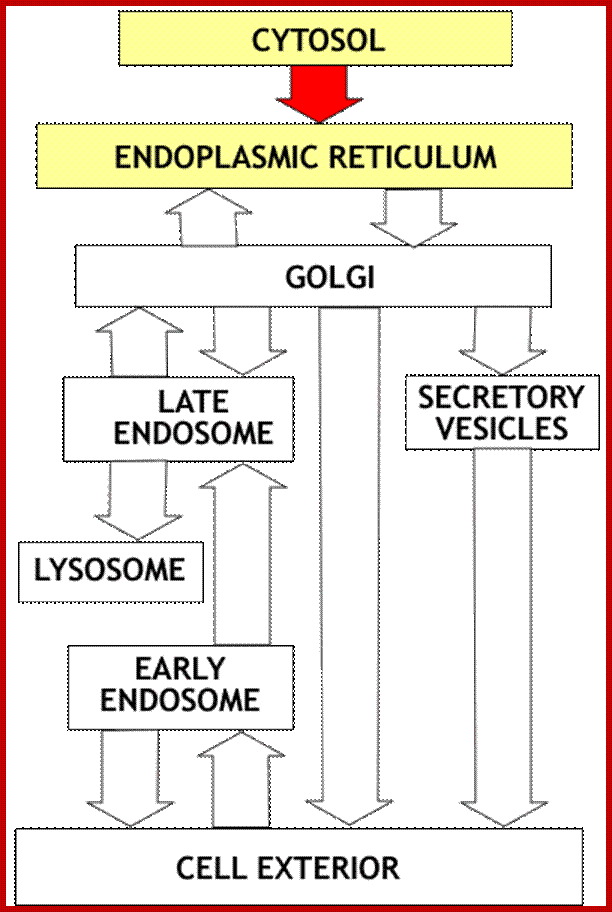
;Vesicle transport and and protein processing;http://www.zoology.ubc.ca/
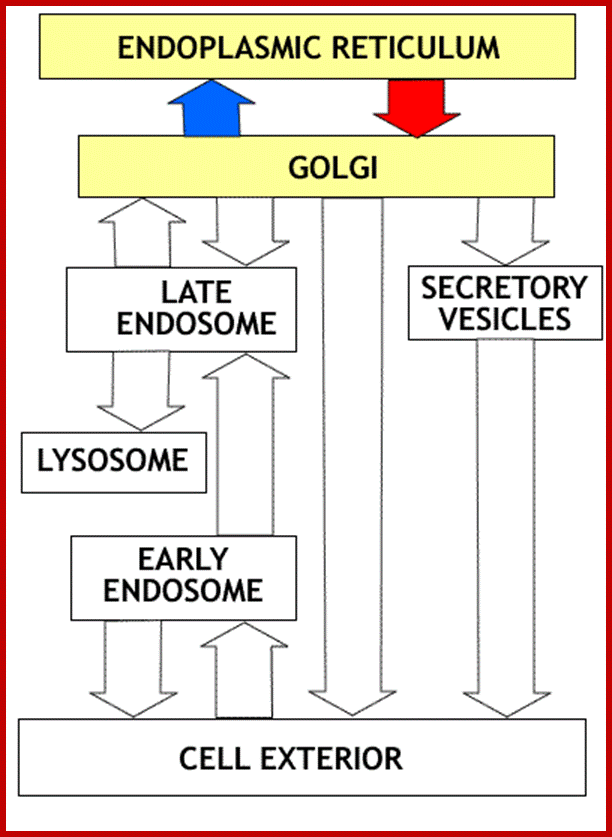
Proteins transfer from ER to golgi and from golgi to different vesicles including lysosme and secretory vesicles, so alo movemen from exterior to early ebdosome, and to late endosome; http://www.zoology.ubc.ca/
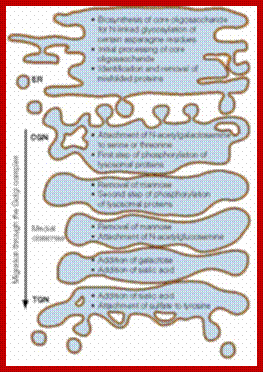
Overview: Synthesis of all proteins begins in the cytosol compartment. For proteins entering the secretory or Lysosomal pathways, the first step is targeting to the endoplasmic reticulum. This targeting relies on a targeting signal encoded in the N terminal portion of the protein. The targeting signal is recognized by a specific receptor that results in the protein entering the endoplasmic reticulum
https://quizlet.com
From SER vesicles containing proteins are budded off and the same join the cis Golgi membranes. There the proteins go through further modification if ne4cessary and as they go through processing such as modifications they reach trans Golgi surface they are then budded off and transported to their respective destinations aided by Microtubules.
http://users.rcn.com/
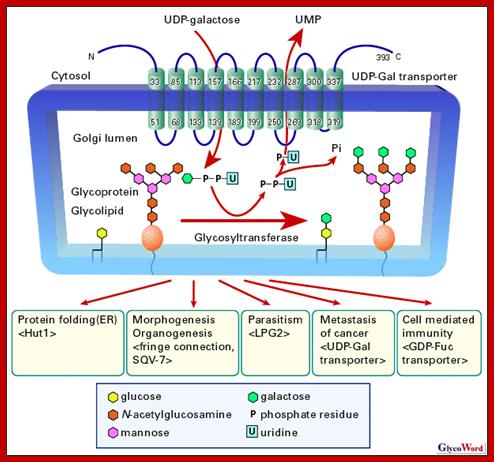
Glycosylation of folded proteins at specific sites or sequences; http://www.glycoforum.gr.jp/

Glycosylation in ER and in Golgi; http://www.zoology.ubc.ca/
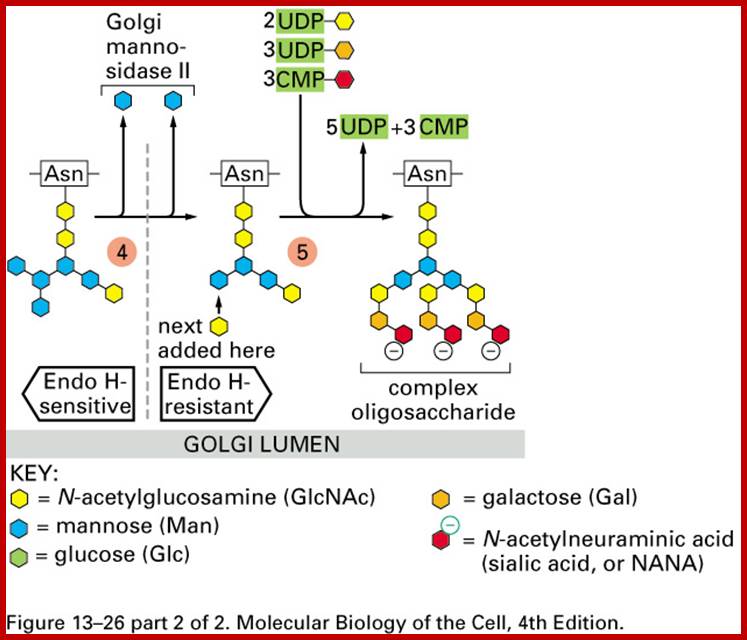
Glycosylation in ER and Golgi http://www.zoology.ubc.ca/
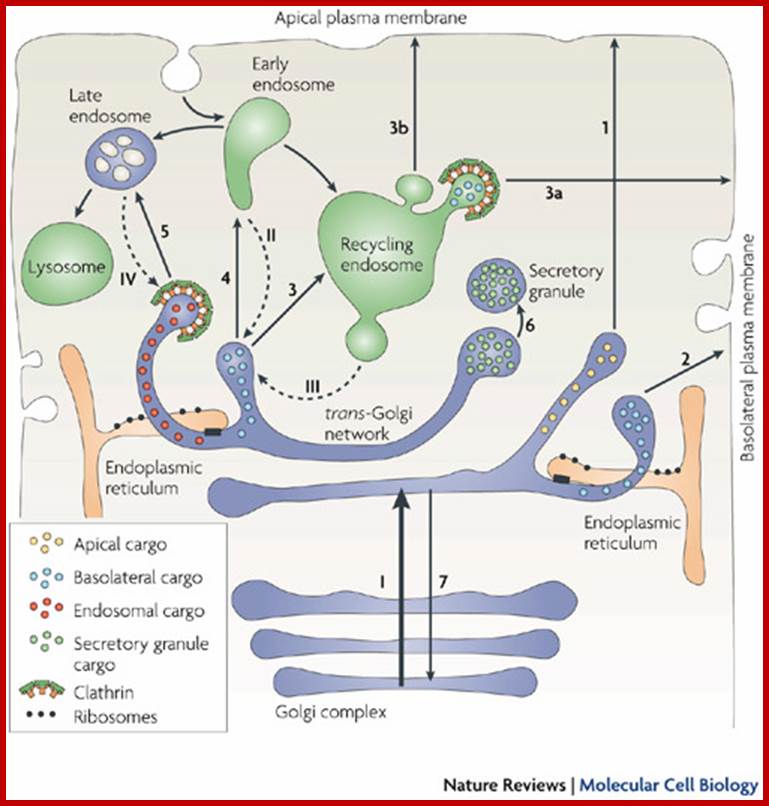
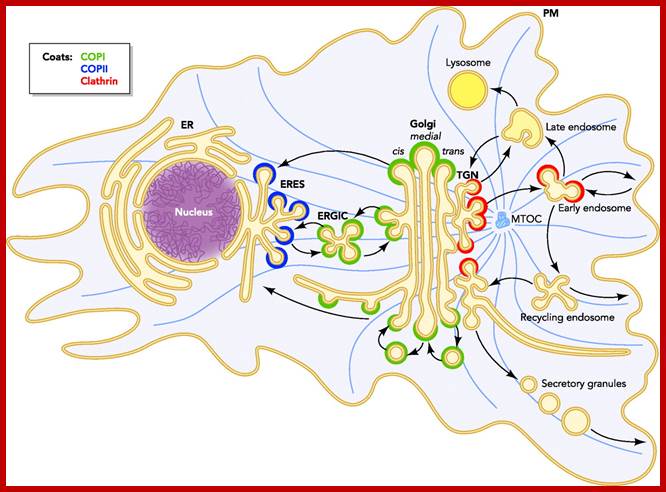
The trans-Golgi network (TGN) is a tubular network that originates from the last trans-Golgi cisternae. It sorts newly synthesized proteins that arrive from earlier Golgi compartments (I) towards different destinations (1–5). It also receives input from the endocytic pathway (II–IV) and sends back components to the earlier Golgi compartments (7). The exit routes from the TGN include those towards the apical plasma membrane (1), the basolateral plasma membrane (2), recycling endosomes (3), early endosomes (4), late endosomes (5) and specialized compartments such as secretory granules (6) in secretory cells. These are the main destinations, and for each of them more than one type of carrier might be involved; for example, basolateral transmembrane and soluble trafficking proteins can use different carriers101, 156. Apical transmembrane proteins and soluble, ciliary and GPI-linked proteins appear to use different pathways. Secretory proteins can also use a transendosomal pathway through recycling endosomes to reach the basolateral (3, 3a) or apical surfaces (3, 3b). Golgi-resident proteins (for example, glycosylating enzymes) recycle back to the Golgi stack , as secretion consumes the last trans-Golgi cisternae. Two important morphological features have emerged from the three-dimensional tomographic studies. First, the TGN is composed of tubules emanating from the last two trans-Golgi cisternae (which are smaller and appear to be detaching from the rest of the stack), although only the tubules deriving from the last cisterna are clathrin coated. Second, the endoplasmic reticulum makes close contact with the last two trans-Golgi cisternae (black boxes), through which lipid exchange can occur between these two organelles.; http://www.nature.com/
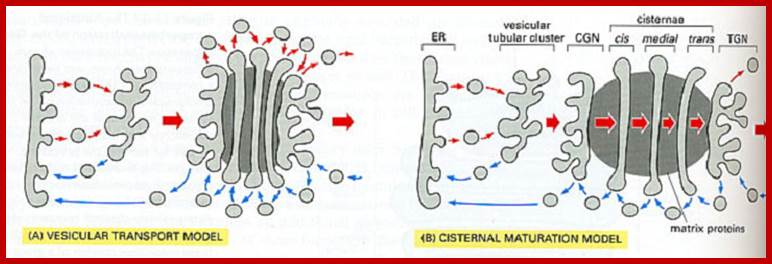
Two models vesicular model and cisternal maturation model; http://greatcourse.cnu.edu.cn/
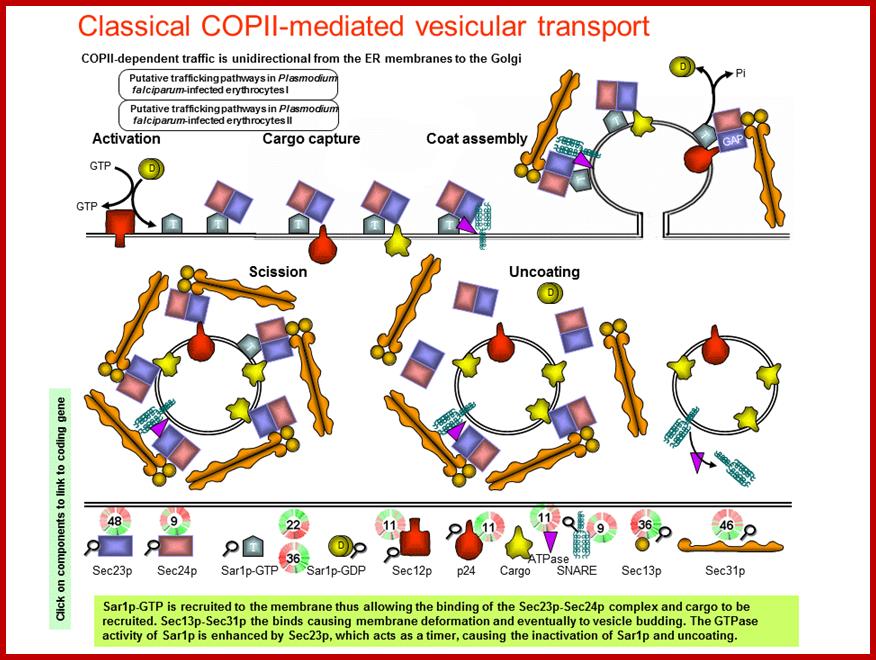
COPII and COPI Traffic at the ER-Golgi Interface; Protein traffic is necessary to maintain homeostasis in all eukaryotic organisms. All newly synthesized secretory proteins destined to the secretory and endolysosmal systems are transported from the endoplasmic reticulum to the Golgi before delivery to their final destinations. Here, we describe the COPII and COPI coating machineries that generate carrier vesicles and the tethers and SNAREs that mediate COPII and COPI vesicle fusion at the ER-Golgi interface ; http://physiologyonline.physiology.org/
Protein traffic is necessary to maintain homeostasis in all eukaryotic organisms. All newly synthesized secretory proteins destined to the secretory and endolysosmal systems are transported from the endoplasmic reticulum to the Golgi before delivery to their final destinations. Here, we describe the COPII and COPI coating machineries that generate carrier vesicles and the tethers and SNAREs that mediate COPII and COPI vesicle fusion at the ER-Golgi interface.
http://mpmp.huji.ac.il/
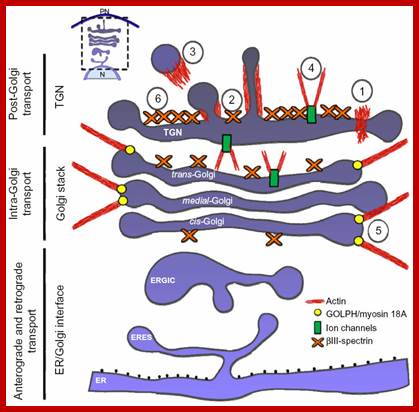
Notes: Diagram of the secretory membrane trafficking pathways and events in which actin is known to participate. Actin filaments, their polymerization, and dynamics could act as a force for the scission (1), pulling (2), and propelling (3) of the transport carrier generated in cisternae, and for maintaining the flattened shape of cisternae through the regulation of the activity on some ion pumps/channels and/or being part of the spectrin-based cytoskeleton (6), and to keep the Golgi ribbon extended. https://www.dovepress.com/cytoskeleton-and-golgi-apparatus-interactions-a-two-way-road-of-functi-peer-reviewed-fulltext-article-CHC
http://physrev.physiology.org/
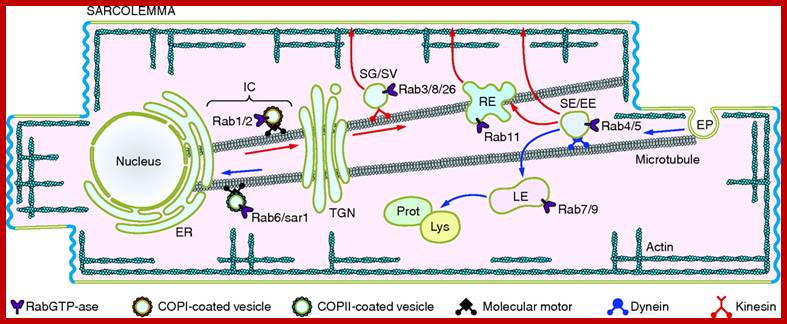
Cardiac Myocytes are characterized by distinct structural and functional entities involved in the generation and transmission of the action potential and the excitation-contraction coupling process. Key to their function is the specific organization of ion channels and transporters to and within distinct membrane domains, which supports the anisotropic propagation of the depolarization wave. This review addresses the current knowledge on the molecular actors regulating the distinct trafficking and targeting mechanisms of ion channels in the highly polarized cardiac myocyte. In addition to ubiquitous mechanisms shared by other excitable cells, cardiac myocytes show unique specialization, illustrated by the molecular organization of myocyte-myocyte contacts, e.g., the intercalated disc and the gap junction. Many factors contribute to the specialization of the cardiac sarcolemma and the functional expression of cardiac ion channels, including various anchoring proteins, motors, small GTPases, membrane lipids, and cholesterol. The discovery of genetic defects in some of these actors, leading to complex cardiac disorders, emphasizes the importance of trafficking and targeting of ion channels to cardiac function. A major challenge in the field is to understand how these and other actors work together in intact myocytes to fine-tune ion channel expression and control cardiac excitability. Elise Balse et al.
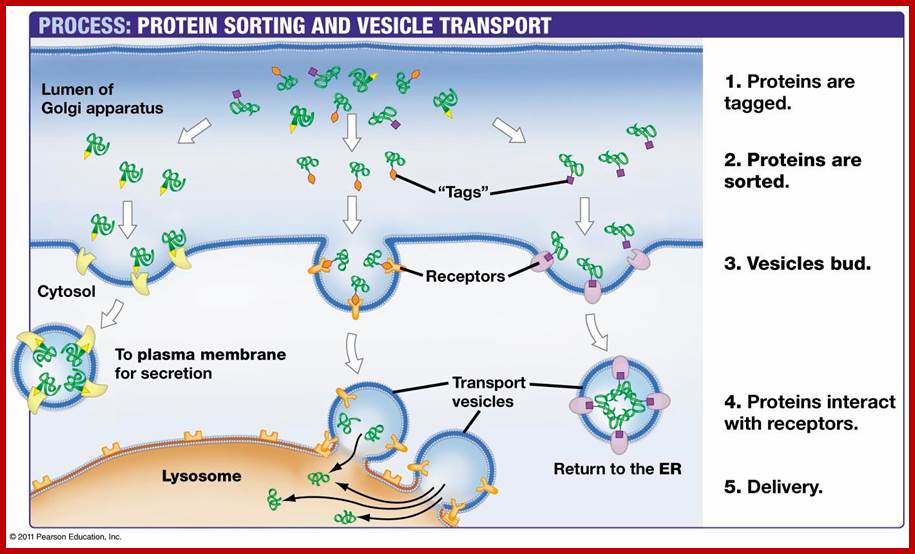
Protein trafficking via membranes; http://web.bio.utk.edu;/http://bio100.class.uic.edu/
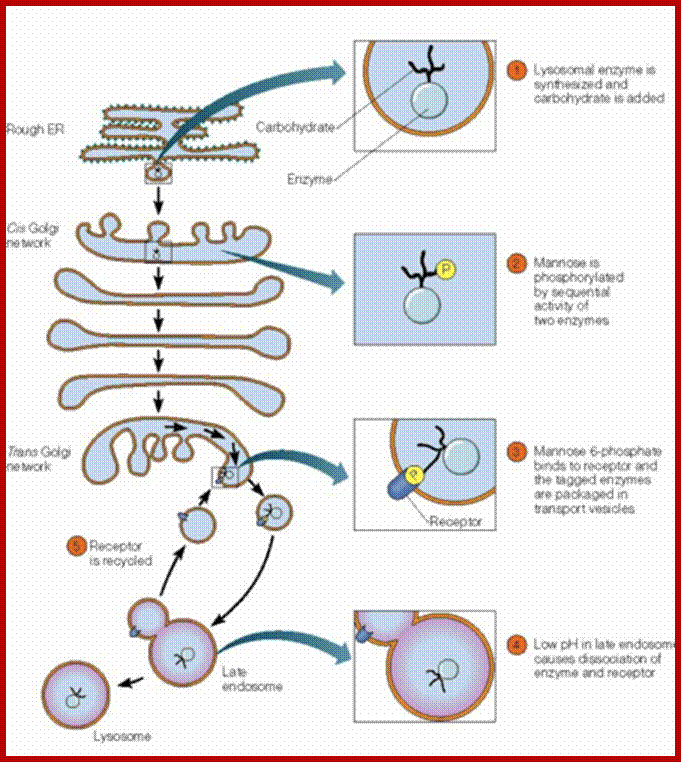
https://quizlet.com/

http://sparkleberrysprings.com/
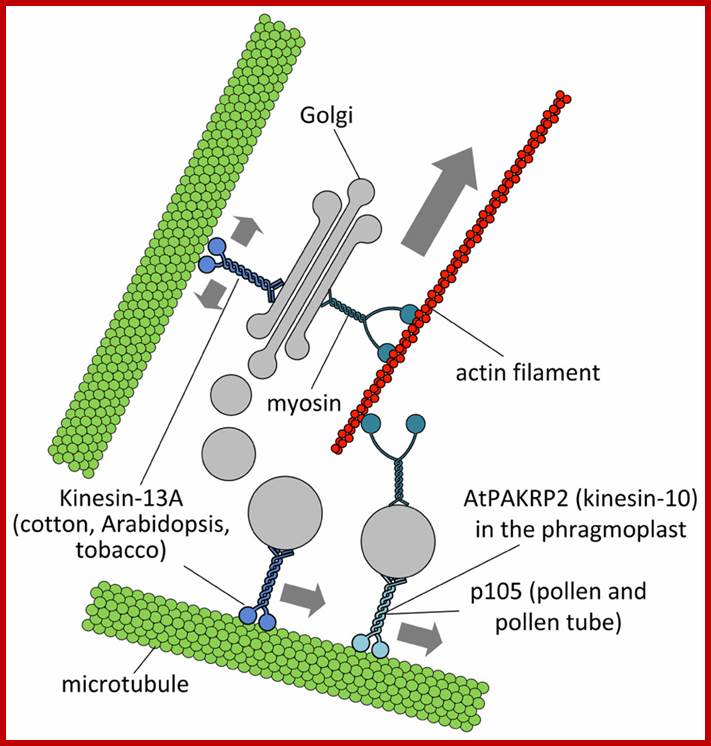
Putative interactions between Golgi stacks/vesicles and motor proteins; Specific subclasses of myosin XI would be responsible for the long-range transport of Golgi bodies within plant cells. Members of the kinesin superfamily are likely involved in either the anchorage of Golgi stacks to microtubules (such as kinesin-13A) or the short-range transport of Golgi vesicles to defined cell positions (such as AtPAKRP2 and p105). The arrow length indicates the relative velocity as produced by motor proteins. Opposing arrows indicate an anchorage role for the motor. http://journal.frontiersin.org/
In plant cells, which do not have centrosome, there are structures resembling a poorly developed Golgi complex, such as small stacks of cisternae or even individual cisternae, all of them dispersed through the cytoplasm. Thus, the Golgi complex looks like divided in many parts distributed through the cytoplasm. Every of these stacks works as independent units, and it is not known if they develop exactly the same functions or not, i.e. if they are specialized in processing of certain type of molecules. Cisternae are smaller than in animal cells, although the total number of cisternae could be from dozens to more than hundred. In plant cells, ERGIC (see below) has not been observed, but the trans Golgi network (TGN) is well developed, so that some authors endorse it as a different organelle
Individual cisternae or dictyosomes can move through the cytoplasm thanks to actin filaments. They move around the places where there is vesicle formation by the endoplasmic reticulum, as if they were collecting new formed vesicles. The morphology of dictyosomes does not change during these movements. Vesicles that must fuse with vacuolas are moved by actin filaments. One more feature of Golgi complex organization in plant cells is that the dictyosomes do not disappear during mitosis, as occurs in animal cells, because Golgi stacks are needed for synthesizing the new cell wall during cytocinesis, which will separate the two new cells. There are some rare Golgi complex organizations in some species. For example, fruit fly, although it has centrosome, shows a Golgi organization similar to that of plant cells. Yeasts, eukaryote cells, do not show any membrane organization similar to Golgi stacks observed in animal or cell plants. http://mmegias.webs.uvigo.es/
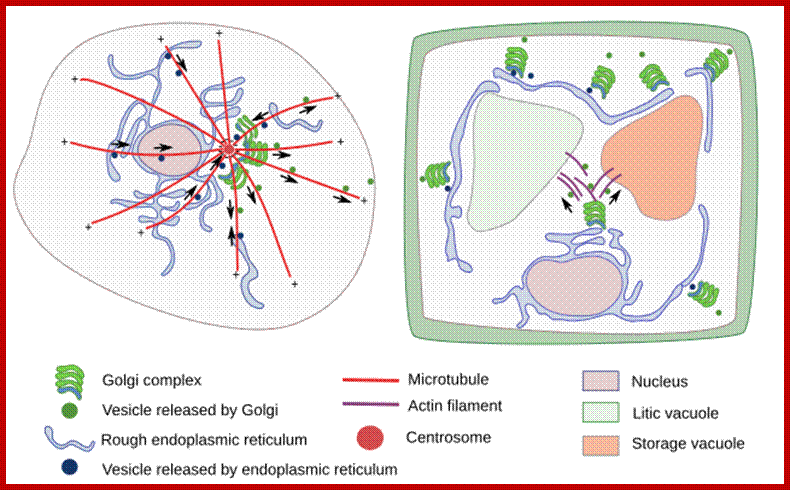
Organization of the Golgi complex in the animal and plant cells. In animal cells, dictyosomes are located close to the centrosome, near to the nucleus, and are organized mainly by microtubules. In plant cells, dictyosomes are distributed through the cytoplasm, they are moved by actin filaments. Arrows indicate the movement direction of the vesicles. http://mmegias.webs.uvigo.es/
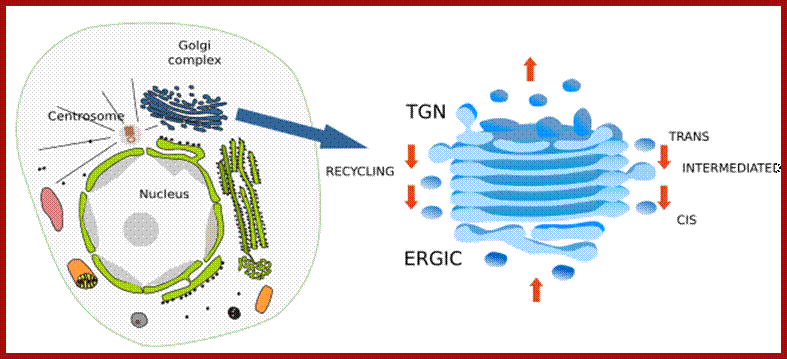
Golgi complex is divided in domains. Cis domain is where ERGIC bodies and vesicles coming from the endoplasmic reticulum fused between each other to form the first Golgi cisterna. ERGIC is not a Golgi compartment, but a transient one between the endoplasmic reticulum and Golgi complex. Cisternae in the middle of dictyosomes are known as intermediate cisternae. Trans domain is where cisternae are transformed in vesicles and tubules containing the molecules to be delivered. TGN is this network of vesicles and tubules. From the lateral part of cisternae, COPI coated vesicles arise and head for endoplasmic reticulum, it is a recycling pathway. Arrows located laterally indicated the direction of the recycling vesicles and arrows located in the center indicate the pathway of the molecules being processed by the Golgi. http://mmegias.webs.uvigo.es/02-english
Golgi complex is a polarized structure, dictyosomes show two domains: cis and trans domains. Intermediate cisternae are located between them. For example, cis cisternae contain higher concentration of N-acetylgalactosamine transferase, intermediate cisternae contain more N-acetylglucosamine transferase I, whereas trans Golgi has more galactosyl transferase and sialyltransferase. In the cis domain, there is an ongoing addition of new material from the endoplasmic reticulum coming as part the ERGIC (endoplasmic reticulum Golgi intermediate compartment) bodies. In the trans domain, there is a tubulo-vesicular arrangement of the membranes, referred as TGN (trans Golgi network), where molecules are distributed in vesicles and tubules, in their way toward different destinations. Thus, there is a traffic of molecules starting in the cis cisternae, going through intermediate cisternae, and ending in the trans domain. There is also a recycling pathway carried out by COPI coated vesicles, which bud from the lateral part of the cisternae and are directed toward the endoplasmic reticulum. The Golgi complex is in permanent renewing and its organization and size is affected by the traffic of molecules. It is particularly well developed in the cells showing a strong secretion.
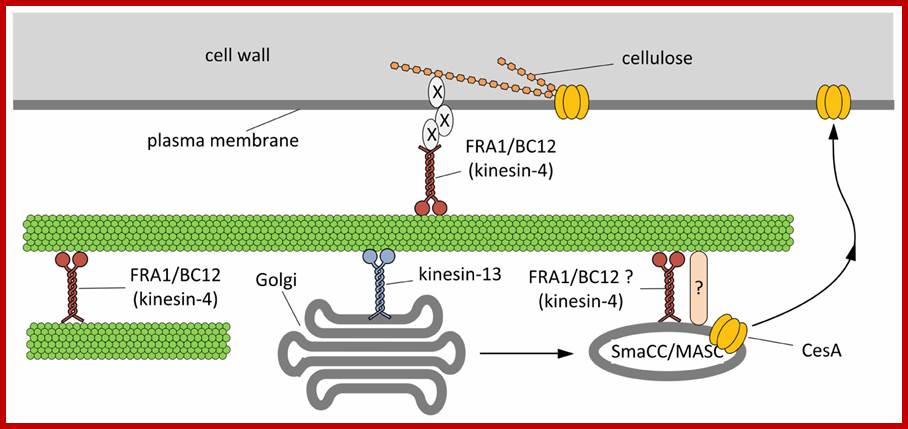
Hypothetical model outlining the functions of kinesins during the assembly of cell wall. On the basis of current literature, members of the kinesin-4 subfamily (FRA1 and/or BC12) might be used to organize microtubules beneath the plasma membrane in order to favor either the proper insertion or the activation of cellulose synthase (CesA). The interplay between kinesins and CesA might be also more direct. Members of the kinesin-13 subfamily have been hypothesized either to transport locally or to pause Golgi stacks along microtubules. Once assembled in the Golgi stacks, CesA might move into the so-called SmaCC/MASC compartments, which are known to interact with microtubules, a step required for the insertion of CesA into the plasma membrane. The proteins mediating the interaction between SmaCC/MASC and microtubules are partially known (indicated by question mark) and FRA1/BC12 might putatively be part of such complex. As a further hypothesis, FRA1 and BC12 might be part of the complex that organize the nascent cellulose microfibrils at the plasma membrane interface by delivering specific components that regulate the orientation of cellulose microfibrils (indicated by X). http://journal.frontiersin.org/
http://www.protein.bio.msu.ru/
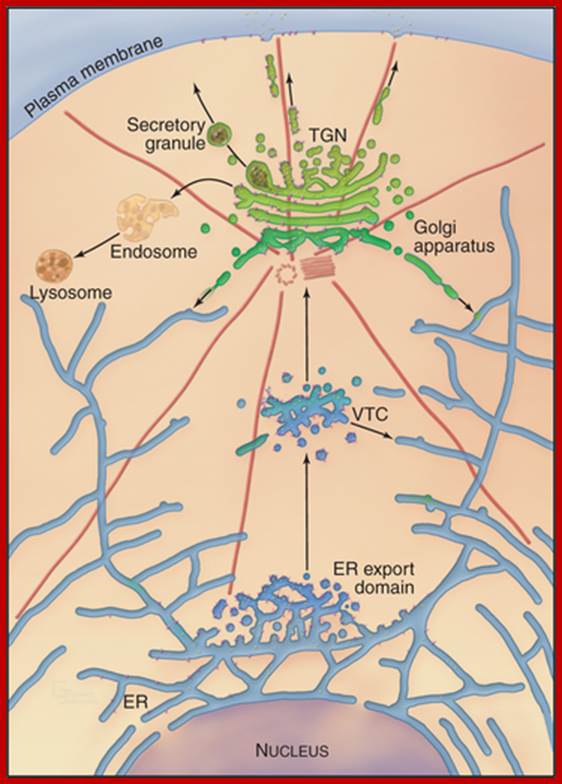
http://clinicalgate.com/
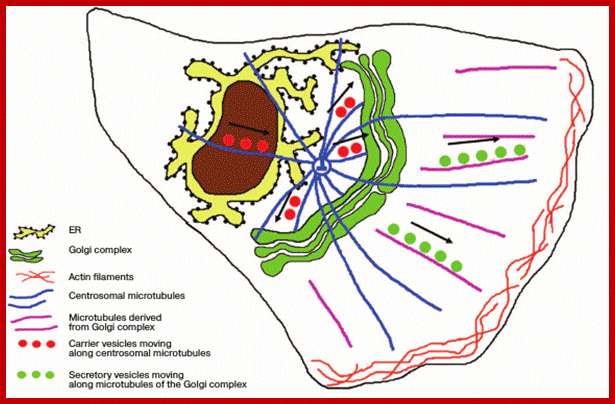
Position of the ribbon-shaped Golgi apparatus with respect to the centrosome: in polarized cells. Products of synthesis are delivered from the ER along the centrosomal microtubules to the Golgi apparatus steadily localized near the centrosome. Secretory vesicles are transferred to the plasma membrane along microtubules derived from the Golgi apparatus.
Number of Golgi Bodies per cell:
It varies from one system to the other, In mammalian cell a single Golgi located near nucleus and associated centrosomes. In maize root caps one finds 300-600 golgi complexes. The stacks are distributed all over the cell a small groups. Golgi bodies are localized in a region a small distance away from the growing tip; found in root hairs and pollen tubes; secretory vesicle movement is inhibited by cytochalasin B for they act of microfilaments. In mammals, a single Golgi apparatus complex is usually located near the cell nucleus, close to the centrosome. The stacks are connected by tubules require microtubules. In yeast, multiple Golgi apparatuses are scattered throughout the cytoplasm (as observed in Saccharomyces cerevisiae).; Organization of the plant Golgi depends on actin cables and not microtubules.[5] The common feature among Golgi is that they are adjacent to endoplasmic reticulum (ER) exit sites. In plant single stacks are found, in animals ten to 40 stacks are organized; the antibody-secreting plasma B cells of the immune system have prominent Golgi complexes.
Microtubules
also have a role. Formation of the cell plate requires intensive secretion of
Golgi-synthesized cell wall polysaccharides. The phragmoplast, a structure
containing microfilaments and microtubules, spatially orients and positions the
new cell wall by directing Golgi-derived vesicles to from the cell plate.
Formation of the cell plate requires intensive secretion of Golgi-synthesized
cell wall polysaccharides. The phragmoplast, a structure containing
microfilaments and microtubules, spatially orients and positions the new cell
wall by directing Golgi-derived vesicles to the cell plate . The cisternal membranes and
associated vesicles can be from 0.5 to 2.0 μm in diameter.
Golgi stacks are usually composed of three to eight cisternae, and may be composed of 15 or more in scale-secreting algal cells. Nevertheless, within a single cell, the cisternal number is remarkably uniform.
Biosynthetic functions of the plant Golgi apparatus;
The plant Golgi apparatus synthesises a wide range of cell wall polysaccharides and proteoglycans, and also carries out O-linked glycosylation and N-linked glycan processing.
The two abundant classes of Golgi-synthesised polysaccharides, the pectins and hemicelluloses, can constitute between 50 and 80% of the dry weight of the cell wall. From the peripheral area of cisternae arise a complex, anastomosing flat network of tubules of300 to 500 A° diameter. http://www.yourarticlelibrary.com/
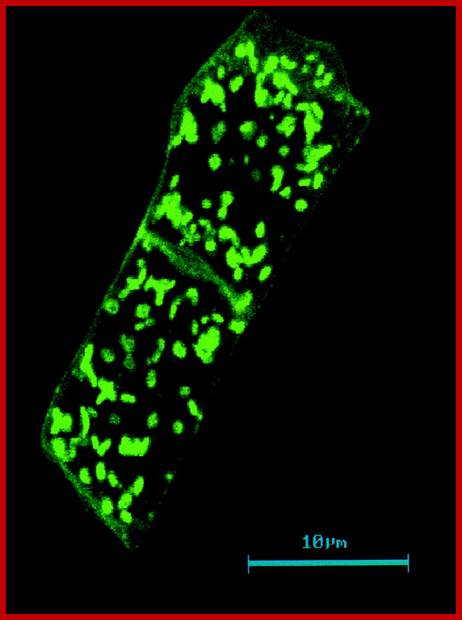
Distribution of Golgi bodies within two Nicotiana root cells shown by labelling with JIM84. The monoclonal antibody JIM84 recognises a carbohydrate epitope on many Golgi and plasma membrane glycoproteins. Golgi bodies are dispersed singly or in small groups; http://www.yourarticlelibrary.com/
Sosa has suggested the following nomenclature for the Golgi complex: 1. Golgiokinesis: Division of the Golgi apparatus during nuclear division. 2. Golgiosomes:
Corpuscles produced by the Golgiogenesis are called as Golgiosomes which are described as Golgi material in invertebrates. 3. Golgiolysis: Process of dissolution of the Golgi apparatus. 4. Gogiorrhexis: Fragmentation on the Golgi apparatus. 5. Golegiogenesis: Formation and differentiation of the Golgi body during embryonic development. 6. Golgio-cytoarchitecture: Study of structure of cell in relation to Golgi apparatus. http://www.yourarticlelibrary.com/
For More refer: www.grkraj.org