FLUID TRANSPORT
Loss of water-II


Under our People-Banyan Tree for Chit-Chat among Retired People; www.davey.com; www.newbuddhist.com
The Hitachi Monkey pod tree; ‘Samenae saman tree’- wide spread 200ft; found in
Yucatan Mexico, http://forestry.about.com/

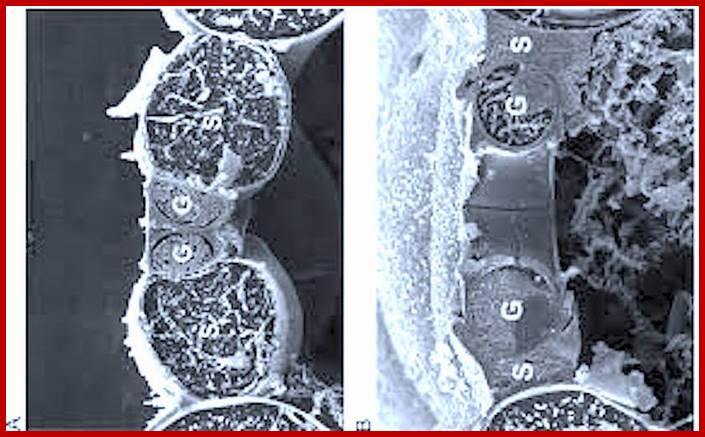
With its large number of aerial roots, the Great Banyan Tree (Ficus benghalensis), Calcutta, looks more like a forest than an individual tree. It is about 200-250 yrs old before the main trunk was cut for it was decayed. It occupies an area of about 14,500 square meters (1.5hectares). The tree now lives without its main trunk, which decayed and was removed in 1925. The circumference of the original trunk was 1.7 m and from the ground was 15.7 m. The present crown of the tree has a circumference of about 1 kilometer, occupies nearly 14,500 square meters 1.5 hectares) and the highest branch rises to about 25 m; it has at present 2880 aerial roots reaching down to the ground. http://www.amusingplanet.com/
Transpiration:
Transpiration is a physiological phenomenon in which water is lost from certain surfaces of the plant body in the form of water vapors. This process is essential for the upward movement of water and mineral nutrients from the water/soil-base. Transpiration along with evaporation of moisture on lands produces 2/3s of the atmospheric moisture that falls as precipitation on land surface. Rest is from sea water (oceans) (https://www.ucar.edu). Based on the structures involved, transpiration has been classified into cuticular, lenticular and stomatal transpiration.
Magnitude of transpiration:
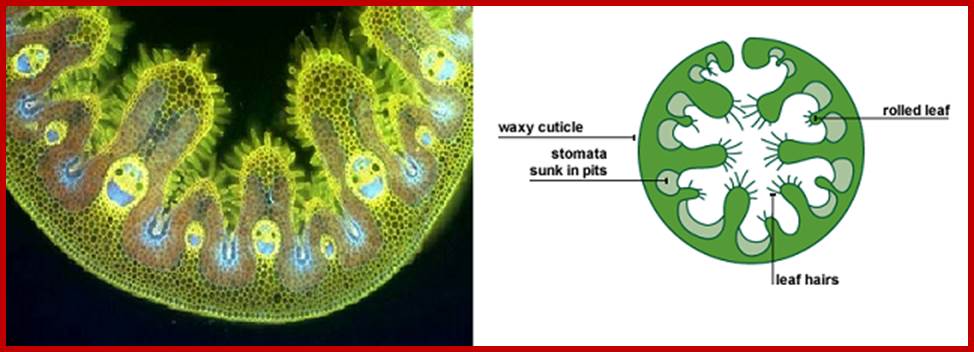
Transpiration along with evaporation of moisture on lands produces 2/3s of the atmospheric moisture that falls as precipitation on land surface. Plants lose most of its absorbed water by transpiration process that too through stomata. However, the total amount of water lost, including all kinds of processes, from a single plant, varies from species to species, which inturn depends upon the structural features of the plant organs involved in transpiration and environmental factors. Larger the tree larger is the amount of water lost by means of Transpiration. Xerophytic plants with their reduced leaf surfaces, thick cuticle, multiple epidermal layers, shrunken stomata, have structurally adopted so as to prevent loss of water to the minimum. But the majority of plants which belong to mesophytic community are so designed, they loose maximum amount of water, in fact they are the plants which deplete most of the soil water.
A corn pant loses about 200 liters of water in one growing season. A single cotton plant looses about one liter of water a day, which is equivalent to 1 cm of rain water per day per acre. One the other hand, corn plants growing in one-acre loose water which is equivalent to 3-4 mm of rain water per day. A single maple tree with a large number of foliage is found to loosen about 100-125 liters of water per hour. An acre of deciduous forests in tropical regions loose about 7,000 to 20,000 liters of water per day. An acre of corn crop can lose 400, 000 gallons of water in a season. A hard wood tree loses 40-100 gallons of water per day. More than 90% of water taken up from soil is lost from plants by the way of transpiration. The above examples indicate that most of the annual rain water absorbed by roots is transpired this way. Environmental conditions that facilitate transpiration- warmer air can hold more water, so it acts a driving force for the water to move out of plant leaves. Wind blowing on the surface can increase the rate of transpiration. Sun light level not so intense can induce stomata to open and loose water. Dry atmosphere is a driving force for greater transpiration. But thick cuticle and wax layers of the leaf surface reduce transpiration. Certain studies indicate evapotranspiration can lose 25% or more water overnight from leaves surface at night. Lysimeter 2m^2 a new technology, where they found that nocturnal water losses in a row of crops monoculture of bean and cotton showed an order of magnitude of nocturnal evapotranspiration at global level 51-98 vs 7-8 mm yr-1 (Victor Resco de Dios et al 2915 ; Nature .com).
www.ujfvalencebio110.files.wordpress.com
CUTICULAR TRANSPIRATION
This process is often called epidermal transpiration for some water is lost through the cuticle found at the outer surface of the epidermal layer of cells. Cuticle is a layer which is made up of complex waxy substances called Cutin. In fact, the cutin is synthesized and deposited by epidermal cells on its outer surface. It is a mixture of free hydroxy fatty acids and soaps. The cuticle layer thus deposited may be thin or thick, but it covers the entire surfaces of the mesophytic plant body including dorsal and ventral epidermal surfaces of leaves. But aquatic plants are lacking in the cuticle.
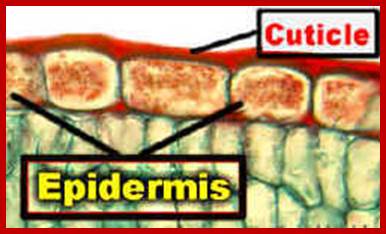
The purpose of secreting cuticle layers is to prevent the loss of water from the surface cells. But the cuticle layers are not continuous, because mechanical stress, brittleness or injury may cause the cuticle to break open here and there as small fissures. It is through these openings; water is lost to atmosphere from the under lying epidermal cells. Still the total amount of water lost is not that significant, in terms of the total amount of water transpired by stomatal mechanism.
Stomatal transpiration
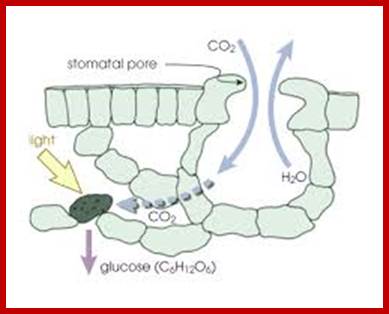
The green leaves, with their vivid shapes and colors, perform many vital functions, such as synthesis of food material by photosynthesis, transport of photosynthate and they also loose water through special structures they possess. The epidermal structures that lose water is called stomatal apparatus. These structures greatly felicitate the exchange of gases, but they are also mainly responsible for loosing more than 90% of the water that is lost from the plant body.
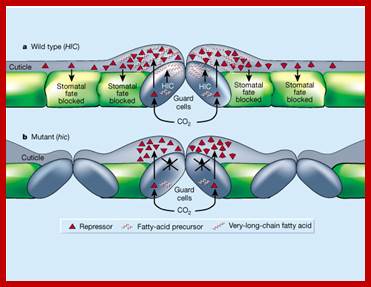
www.lima.ohio-state.edu; http://www.fao.org/
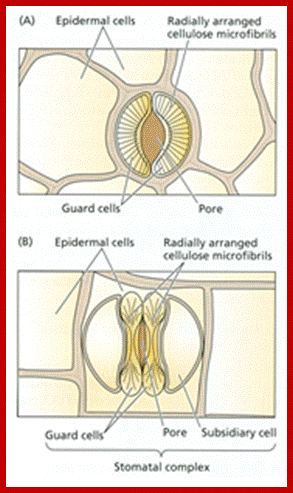
Top dicot and lower Monocot stomata; http://www.tutorvista.com/http://www.tankonyvtar.hu/; The radial alignment of the cellulose microfibrils in guard cells and epidermal cells of (A) a kidney-shaped stoma and (B) a grass like stoma (source: Taiz L., Zeiger E., 2010)

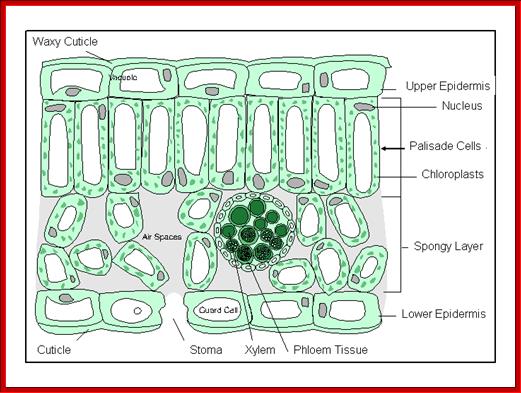
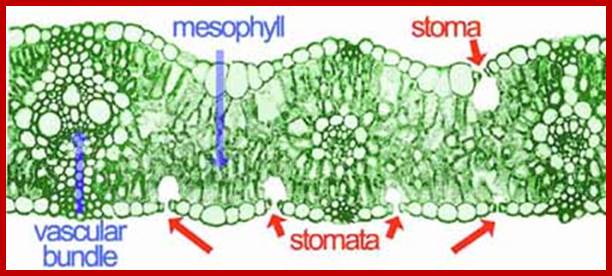
http://sci.waikato.ac.nz/
www.study.com; www.dreamstime.com
![]()
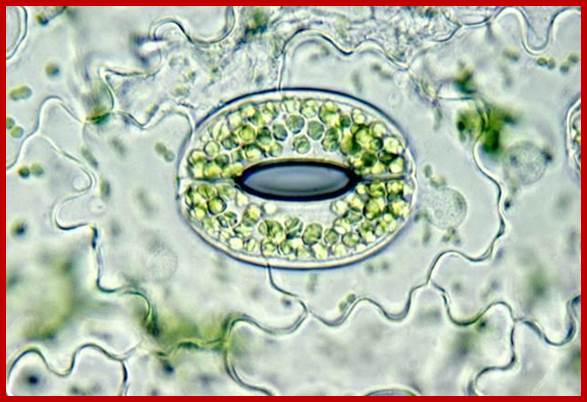
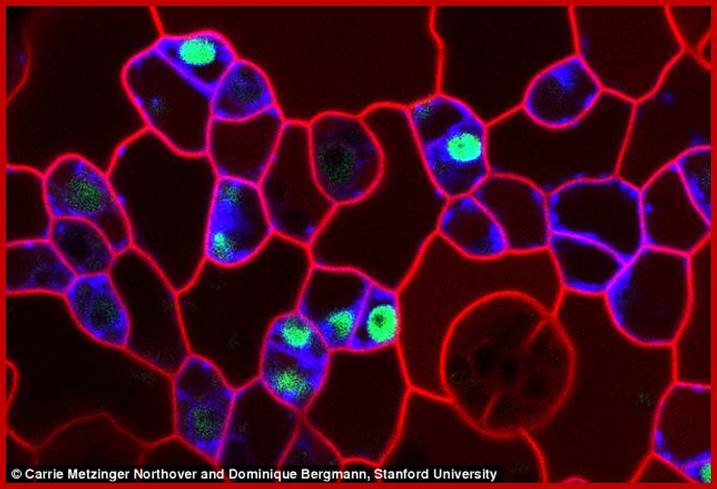
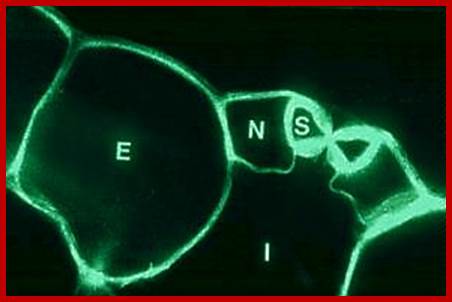
Many, many chloroplasts in Guard cells; https://www.studyblue.com
Guard cells contain chloroplasts and mitochondria with other cell organelles like Golgi, ribosomes and other cell organelles, the cell is phosynthetically very active e and responds sunlight; But subsidiary cells lack chloroplasts , so they respond to light, but contain a large central vacuole; http://mcdowellscienceexam.weebly.com/plants.html
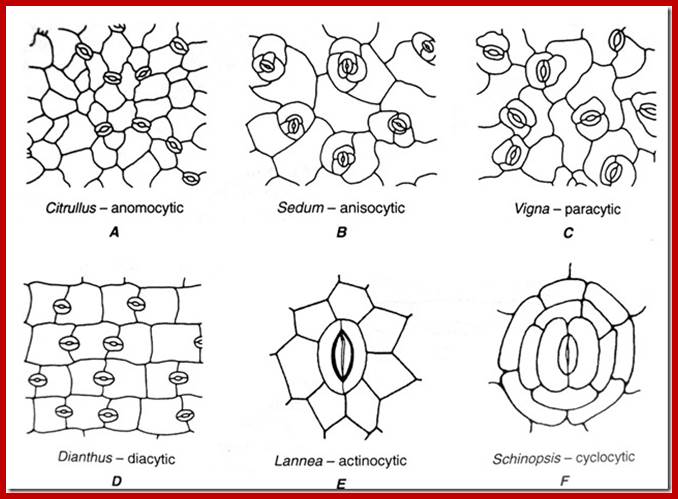
STOMATAL TYPES AND DISTRIBUTION
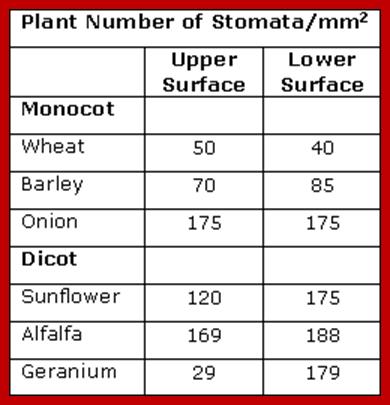
Stoma is a pore and the term stomata refer to pores with adjoining cell structure is called stomata. Surface stomata and sunken or buried stomata as they are the epidermal openings, they are found in both dorsal and ventral epidermal layers, but their distribution pattern varies from species to species. Though the distinct pattern of distribution of stomata is determined by its genome, environmental factors like shade, sunlight, and habitat have a profound influence on the distribution pattern of stomata. Nonetheless, based on the relative distribution pattern between the dorsal and ventral surfaces of the leaves, five groups of plants have been identified. In the recent years, the stomatal distribution pattern has also been used as one of the parameters for identifying and placing the species in a particular group.
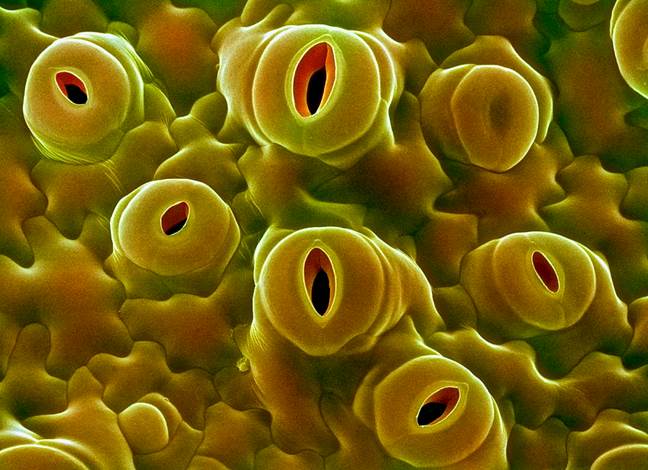
www.asknature.com; www.fineartamerica.com
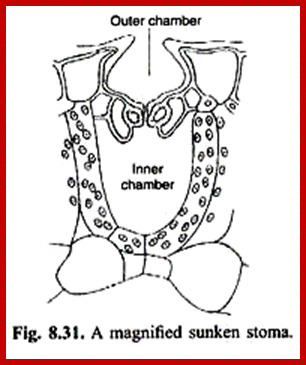
Sunken stomata, inner chamber; www.nasa.gov
Sunken stomata, outer surface: https://vcebiology.edublogs.org
http://biology-igcse.weebly.com/
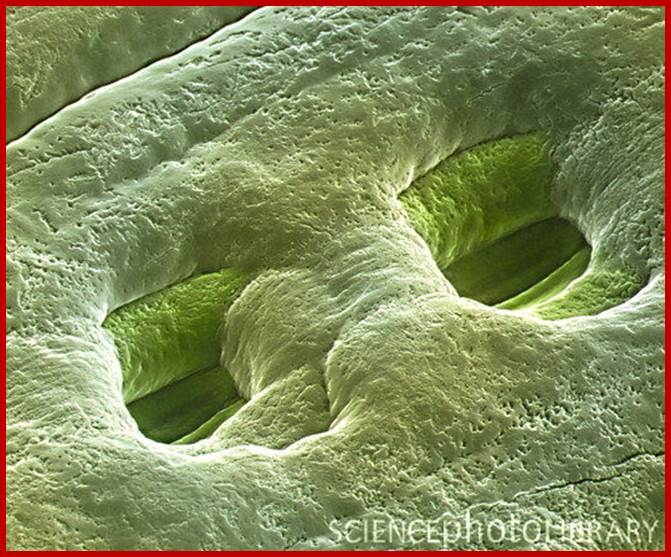
http://www.sciencephoto.com/
http://imgarcade.com/
1. Apple / Mulberry Type: In this category of plants, stomata are present only on the ventral surface and the stomata are almost absent on the dorsal surface. Ex. Quercus, Acer, Apple, Mulberry, etc.
2. Potato Type: Plants belonging to this category contain stomata distributed on both the surfaces but greater number is found on the ventral surfaces than the dorsal surface. Ex., Tomato, Potato, Cabbage, Brassica, Helianthus, Pea, Beans etc.
3. Oat Type: Most of the plants belonging to grass members have isobilateral leaves, because of their equal disposition to light and shadow. They contain equal number of stomata on both the surfaces. Such distribution is not just restricted to only monocot grass members, but also found in many groups of dicot species; which suggests a common gene pool for expressing their characters; ex. Oat, Rice, Wheat, Eucalyptus, etc.
4. Nymphea Type: Certain aquatic plants like Nymphea, Nelumbium produce leaves which float on the surface of water where the dorsal surface is exposed to light and the ventral surface is in contact with water. The leaves belonging to such category contain greater number of stomata on the dorsal side than the ventral surface. Ex. Vioctoria regia, Nymphea, Nelumbium etc.
5. Potomegation type: Potomegaton is an aquatic plant. The leaves of these plants contain stomata which are non functional. No environmental factor can induce them to be functional as the other stomata. Hence, such ineffective stomata are called vestigial stomata. Ex., Pot megaton, Blixa, Vallisneria, etc.
Classification by Metcaffe and chalk 1950:
http://www.plantscience4u.com/
STOMATAL FREQUENCY AND STOMATAL INDEX
The number of stomata found per unit areas is called stomatal frequency. But if the total number of stomata present is compared to the total number of epidermal cells in a given unit area, one finds a remarkable correlation. This correlation can be expressed as stomata index.
The equation for determining the stomatal index (1) is, 1=S/E+Sx100
Where S=the number of stomata, E=total number of epidermal cells in a unit area of the leaf. Oats contain 54,000 per one square inch, but cucumber contains 428,000 per square inch. A study on stomatal index of leaves at different positions and different areas in the same leaf and plant provides very interesting information about the structure, frequency, distribution and ultimately their function.
STOMATAL EFFICIENCY
Leaves having a large number of stomata, virtually act as wet surfaces which are comparable to porous clay pots filled with water. Through the pores water escapes as vapors in all directions; as a result, water vapor shells develop over the pores. When the amount of water lost from a single large surface area is compared with the amount of water lost through very minute pores found in clay pot; it shows that the latter looses water more efficiently than the former. This is because a perimeter of all small pores in a given area is greater than the total surface area of large opening. The diffusion shells overlap with each other, because of close proximity, still stomata loose water very efficiently. This is because the sum total of all stomatal perimeters is greater than the circumference of the whole leaf. This happens despite the total area of all stomata put together occupies just 1 to 2 % of the total surface are of the leaves. In clay pot, the loss of water from its surface has cooling effect on water that is why people store water in clay pots in summers (homemade refrigerator).
STOMATAL APPARATUS
Development:
In Arabidopsis, three basic-helix-loop-helix (bHLH) genes control the successive steps that lead to stomatal formation. SPEECHLESS (SPCH) drives the cell division that initiates the stomatal cell lineage, MUTE induces the formation of the immediate stomatal precursor cell, and FAMA causes the stomatal precursor cell to divide into the two guard cells. Recent results demonstrate that these genes share functions with their grass homologs, and that MUTE is expressed later in development than its grass counterparts. Other differences in stomatal development between these two plant groups are exemplified by the PANGLOSS1 (PAN1) gene of maize. PAN1, which encodes a Leucine-rich repeat receptor-like kinase with an inactive kinase domain, promotes polarization of the subsidiary mother cell and orients its cell division plane. Because such events do not exist in Arabidopsis, it is likely that the PAN1-like genes of Arabidopsis and PAN1 are paralogs. Together, these results indicate that distinctions in the regulation of gene expression and protein function are both responsible for the divergence of stomatal development between Arabidopsis and grasses. Int. J. Dev Biol.
Stomatal mother cells; http://www.dailymail.co.uk
Stomatal precursor cells are meristemoid mother cells, these in turn produce meristomoids which in turn produce guard mother cells. The first two types of cell divisions are assymetric but GMC goes through symmetric cell division.
They are closely related basic helix loop helix genes, SPCH, MUTE and FAMA are expressed sequentially at each key step to direct cell fate transition during stomatal development. Besides the above said stomata one finds floral nectatory stomata for example in Vicia faba and many such plants which secrete nector.
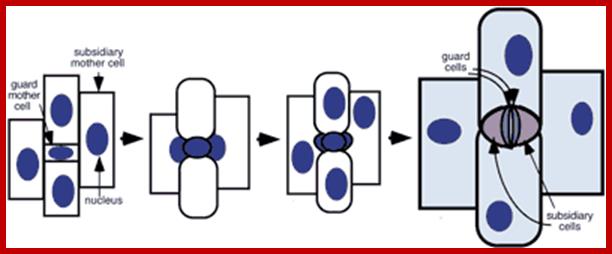
Formation of stomatal complexes in grasses; Following an asymmetric division giving rise to a guard mother cell, the flanking subsidiary mother cells become polarized and divide asymmetrically to form stomatal subsidiary cells; Plane of cell division is always perpendicular to the long axis of the mother cell. Follow Hofmeister's and Errera's rules (Lloyd, 1991). http://6e.plantphys.net
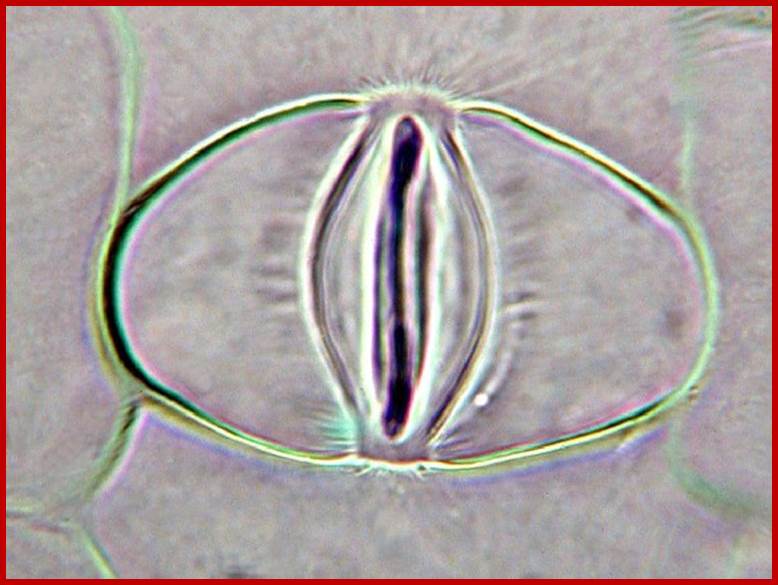
The pore present in the epidermal guarded by two cells is called stoma; the plural form is stomata. Such pores are not just openings, but each pore is surrounded by a set of epidermal cells which are highly specialized in their structure and function. Such a structure is called stomatal Apparatus.
Top- dicot stomats, bean shaped guard cells and lower Few monocots- dumbel shaped guard cells.; http://plantsinaction.science.uq.edu.au
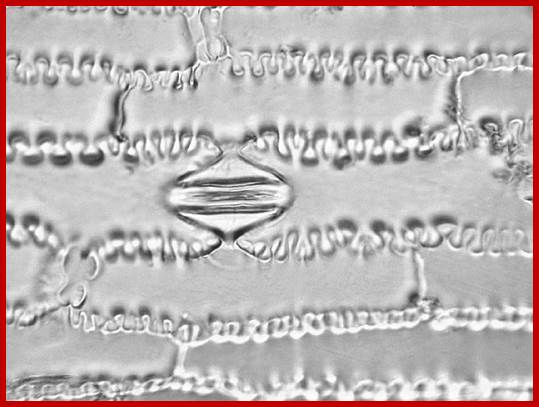
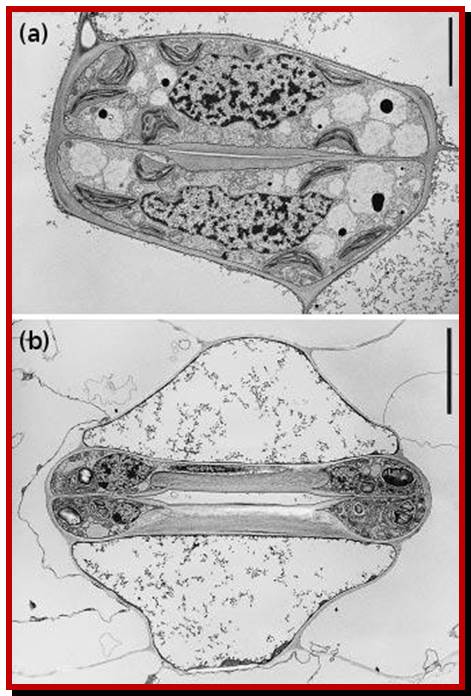
A microrelief of the upper and lower epidermis of
the Tradescantia leaf (Tradescantia sp.); The stomata contain two guard
cells surrounded by subsidiary cells (down). The long ends of guard cells are
look like sealed with some fibers; thus, guard cells can expand only
horizontally, but not longitudinally; (?). Photomicrograph, prim. magn. 100–400×.http://botany.cz/; www.plantbiologyblog.wordpress.com
https://plantstomata.wordpress.com/2015
Guard cells look like sealed at their long ends and thick walls at their ventral walls. https://plantstomata.wordpress.com
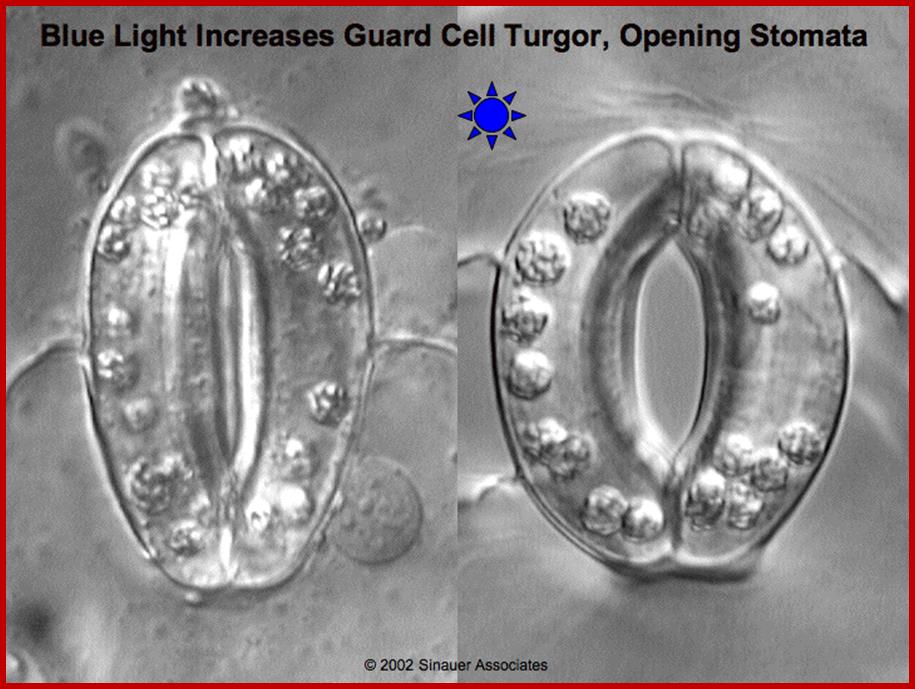
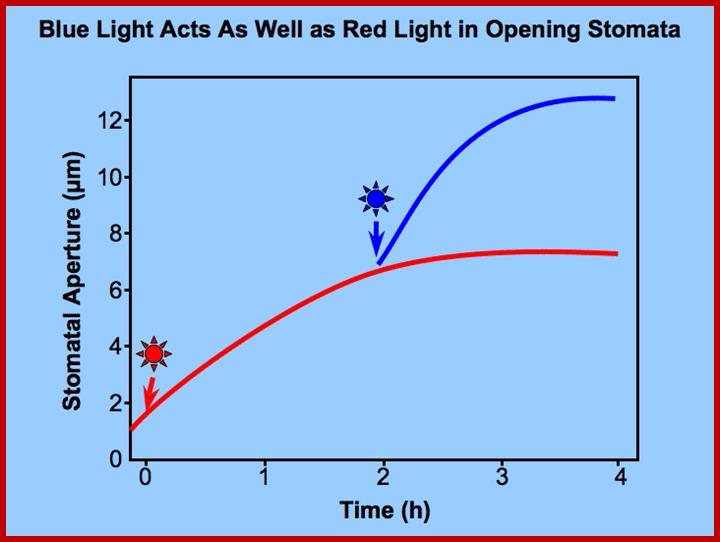
Blue light activates opening of stomata; http://plantphys.info/
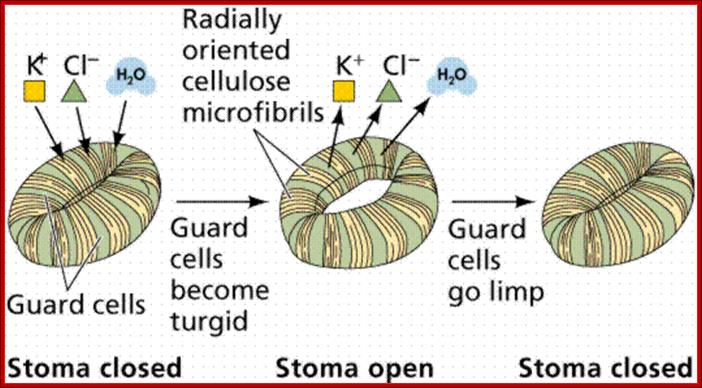
Guard cell are thickened at the inner surface and thin walled at outside , but they are lined with cellulase microfibrils running cross wise which give support to the opening and closing of the guard cells; www.s10.lite.msu.edu
One can observe celluloe microfibrils crass radially and provide strength to guard cells for lateral expansion; http://www.hydroponicsx.com
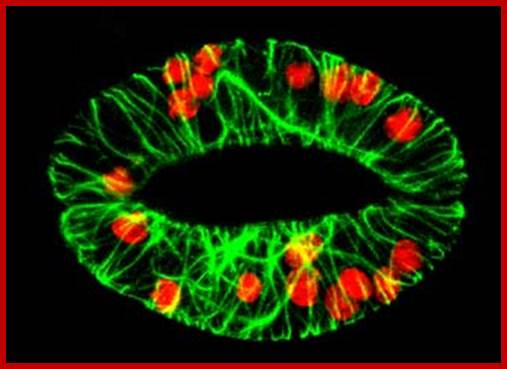
Cellular microfibrils traverse across the guard cells. Cortical microtubules not visible, but with microfilaments radially arranged; they change the pattern during day time and in dark period. http://www.ucd.ie/
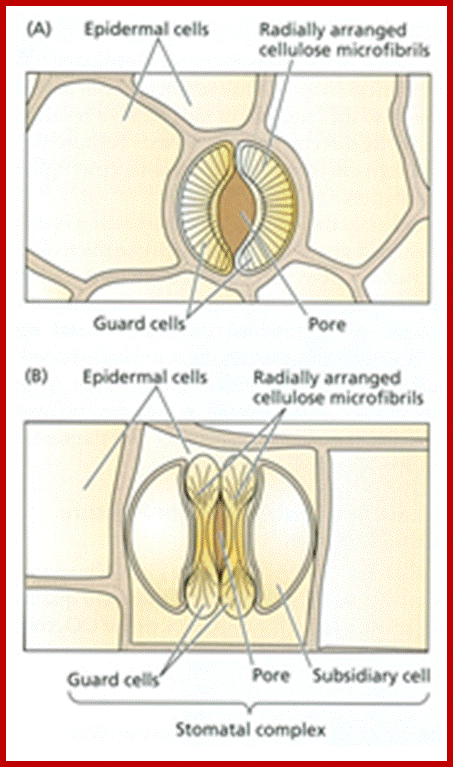
http://www.tankonyvtar.hu/; The radial alignment of the cellulose microfibrils in guard cells and epidermal cells of (A) a kidney-shaped stoma and (B) a grass stoma with enlarged terminal regions and narrower middle region reinforced with thick walls; one can observe cellulose microfibrils at bulbous ends and also all along the length of the guard cells. (source: Taiz L., Zeiger E., 2010)
http://www.sciencedirect.com/
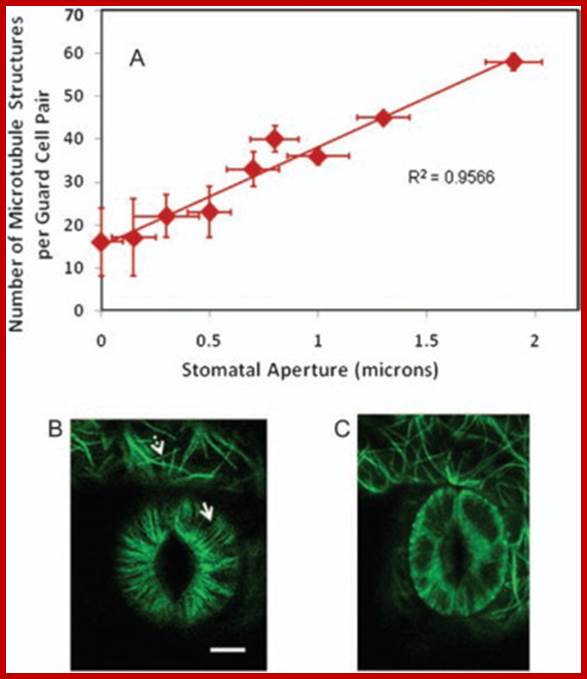
Correlation between Guard Cell Microtubule Structure Number and Stomatal Aperture:
The number of resolvable guard-cell microtubule structures is positively correlated with stomatal aperture for randomly selected greenhouse-grown plants (A) (n = 18). Error bars are standard deviations. Bar = 10 microns. Confocal image of GFP: tubulin-labelled guard-cell pair with open stoma (B) or closed stoma (C). Microtubules (fluorescent images) in guard cells are radially arranged (solid arrow) but fluorescent images in surrounding pavement cells are more random (dashed arrow). Biswapriya B. Misra et al. http://www.sciencedirect.com/
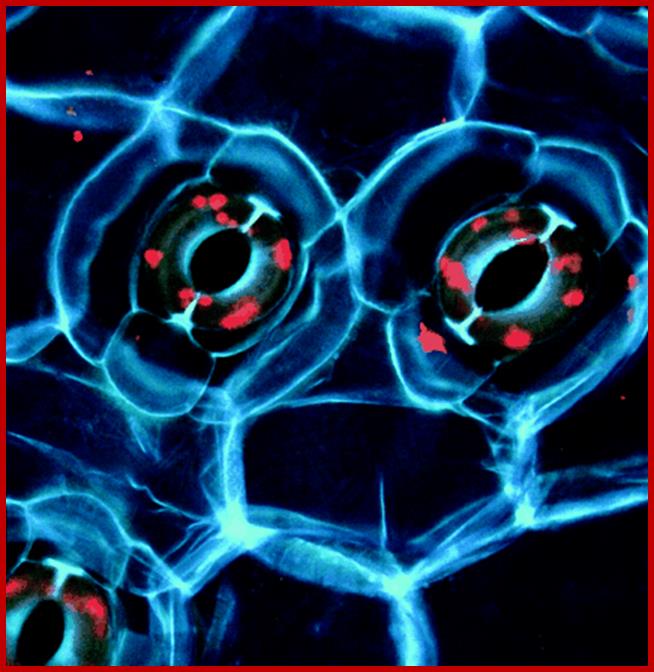
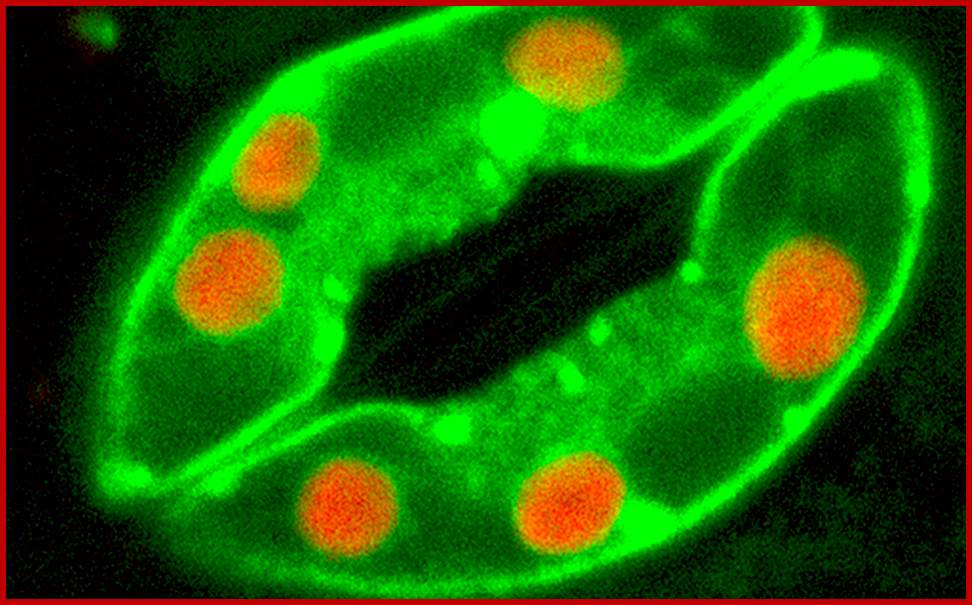
Cover photograph: Pair of stomatal guard cells in the leaf epidermis of the plant Commelina communis viewed under UV light. Cell walls emit blue fluorescence due to phenolic compounds. Red fluorescence is emitted by chlorophyll. The guard cell wall polysaccharide Arabinan pectins play an essential role in stomatal closing, which is important for gas and water exchange. http://www.pnas.org/See the article by Jones et al. on pages 11783–11788.
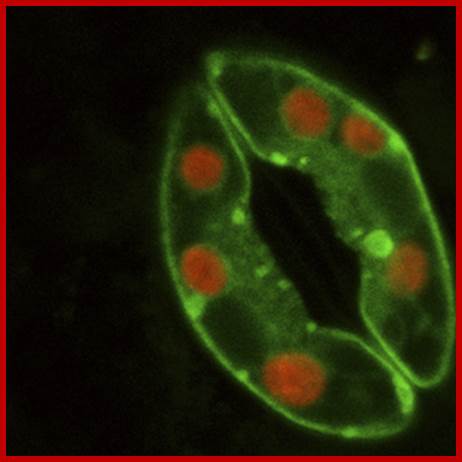
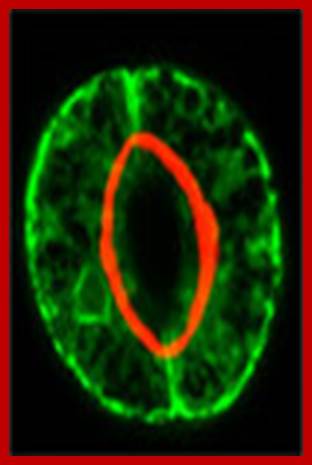
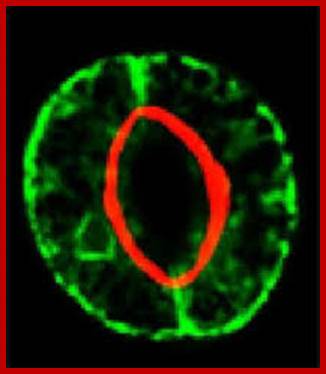
Confocal microscopy image of an Arabidopsis thaliana stoma showing two guard cells exhibiting fluorescence from green fluorescent protein and native chlorophyll (red); http://www.thefullwiki.org/
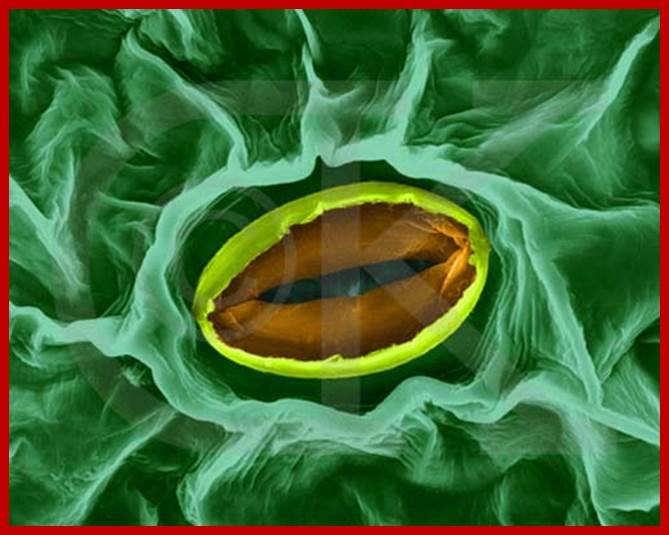
wwwmanythings2know.blogspot.com
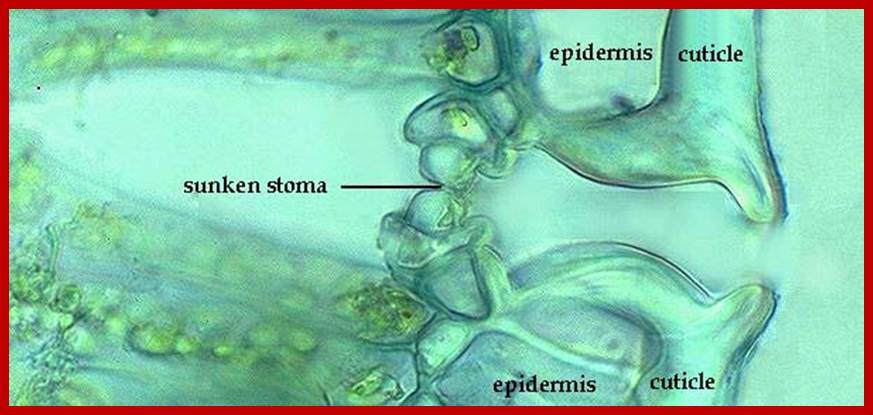
www.sydney.edu.au
In fact, each stomatal apparatus develops from a single epidermal cell; after mitotic division the middle lamella between the daughter cells gets digested and disappears. This is a highly programmed process for no other cells are affected. To begin with these two guard cells were connected with plasmodesmata but once developed no plasmodesmata even with their subsidiary cells? Microtubules and microfilaments are radially oriented their role is not well founded yet treatment with colchicine, cytochalasin and phalloidin show the presence of the said protein elements. However, with cell wall thickened and the disappearance of the middle lamellae, an opening is formed between the daughter cells. The inner walls of each cell get more thickened. The two daughter cells present on either side of the pore are called guard cells, which further undergo differentiation and specialization. The cell walls of the guard cells facing pores become thicker and some of the deposition of additional cell wall material radiates from the inner wall towards the opposite wall, but the cell wall found at the other side of the guard cells, remains thin. The epidermal cells found around the guard cells are called subsidiary cells. They are connected to each other by plasmodesmata in the beginning but later disappear. Microfibrils of cell wall are oriented radially that leads to considerable thickening of inner cell wall of guard cells, but such thickenings are not found between guard and subsidiary cells, surprising fact is the absence of plasmodesmata between them but now it is established that plasmodesmata are found between them. To begin with cellulose microfibrils are oriented in radial fashion; this is almost parallel to microtubules.
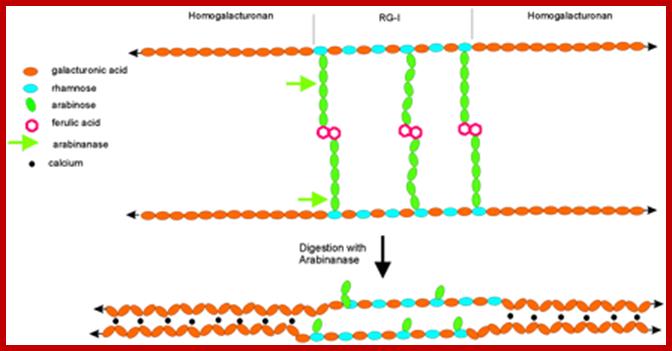
Arabinans in pectins provides fluidity. Arabinans side chain of RG-1 provide steric hindrance to the association of neighboring domains of HGA; arabinans provide flexibility. http://www.pnas.org/
But in monocots example maize and Phleum pretense (timothy–grass) the cells are elongate and bone or dumbbell shaped. The midzone of these cells is thickened and they are thin walled. In grass members differentiation of guard cells is accompanied with the formation of subsidiary cells. When fully formed subsidiary cells surround guard cells and they are larger in size than guard cells. The wall thickening accompanied with the increased activity of golgi apparatus for they provide protein loaded vesicles required for cell wall thickening. Microtubules are implicated in cell wall deposition. The guard cell walls consist of cellulose and also contain other polysaccharide such as pectin and mixed b-glucans and xylans. Guard cells wall are rich in pectins about 30% of the dry weight large amounts of molecules bearing terminal fucose were located predominantly in ventral and lateral guard cell walls. Much smaller amounts of pectins were detected in dorsal walls of these cells. Pectin’s are composed of a mixture of linear and branched polymers characterized by the presence of acidic sugar residues (galacturonic acid) in their backbone, which allows them to form complexes by electrostatic interactions through calcium ions. Linear chains of (1–4)-α-D-galacturonic acid (homogalacturonan) form a major component of pectin’s, and these can associate to form rigid structures. The thick inner wall has a role play in opening and closing of the stomatal pore.
In most of the dicot members, guard cells appear as kidney shaped structures, but in monocots they look like dumbbell shaped structures (not all but general), where the elongated ends are thin walled and flexible and the middle region consists of thick wall. The pore size of the stomata varies from species to species.
Unlike other epidermal cells guard cells are specialized. They contain functional chloroplasts 5-6 in numbers, where as other epidermal cells are totally lacking in functional chloroplasts; guard cells possess well defined nucleus, mitochondria and other cell organelles typical of any other plant cells. Both the ends of guard cells are tightly sealed. Furthermore, subsidiary cells and underlying spongy parenchymatous cells are in contact with guard cells through plasmodesmata, which felicitate the movement of cytoplasmic components from one cell to the other. The presence of starch granules in guard cells during night and disappearance of the same at day times is another important feature of guard cells. Guard cells are protected by larger and thin-walled subsidiary cells which are found on all the four surfaces of the guard cells. Subsidiary cells are in contact with guard cell by thin cell walls and plasmodesmata. Subsidiary cells are metabolically active but lack chloroplasts but contain other cell organelles. They are symplastically connected with guard cells by plasmodesmata.
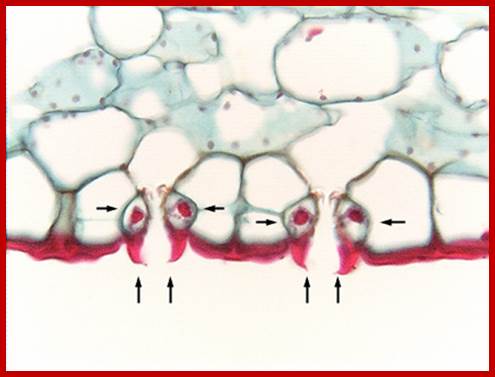
http://plantstomata.wordpress,com
A microrelief of the epidermis of the Cattail (Phragmites australis) and Corn (Zea mays). The cells are indented in a zipper-like fashion. Photomicrograph, prim. magn. 200×.
Subsidiary cells, not only larger than guard cells but act as store house of various components required for guard cells to function. They are not only found on all the four sides of guard cells but the cover stomatal openings by projection as shown in the figure. They show indentations similar to zipper-like fashion.
Factors that control stomatal movements:
LIGHT
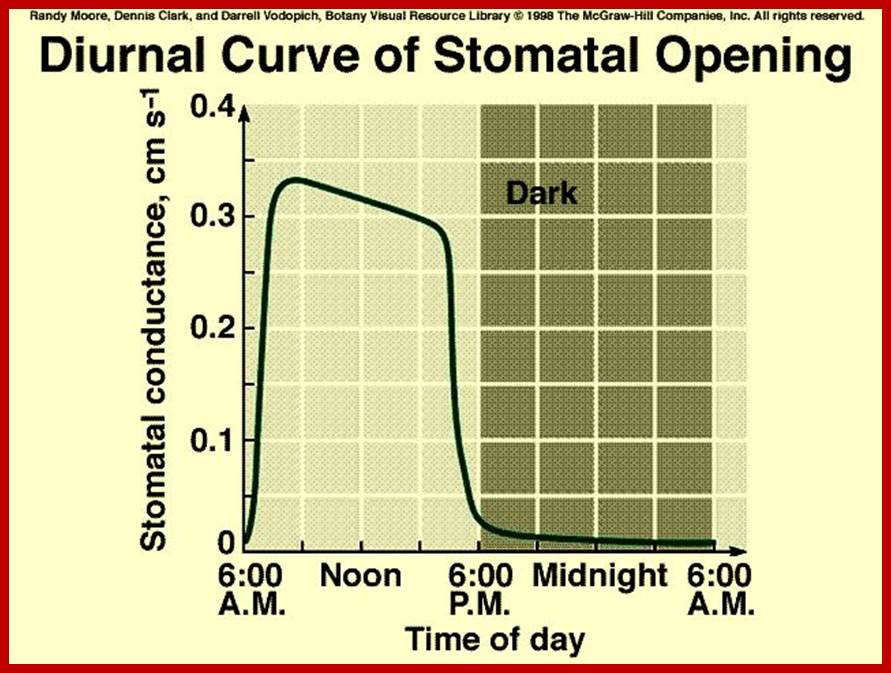
Opening and closing of stomata exhibits diurnal rhythm, in the sense stomata open during day time and close at nights. So light has an important role in this process. The analysis of action spectrum reveals that blue light and red bands together are very effective in the opening of stomata. Incidentally the same action spectrum is also active in photosynthesis. Finer analysis shows that blue light is more effective in opening of the stomata than red light.
TEMPERATURE
As temperature provides energy for the movement of cellular components within the protoplasm; its decrease or increase has a profound effect on the cellular metabolism. Guard cells are no exception to this rule. At low temperatures such as 00 C degree to 8 0C, stomata remain closed, but with the increase in temperature from 8 to 30 0C or so, stomata open and further show a temperature coefficient equal to 2. However, high temperature has contrary effects, and induces the closing of stomata.
CONCENTRATION OF CO2
Correlative studies between the concentration of CO2 and opening closing of stomata reveal that higher concentration of CO2 induces the closing of the stomata even during day conditions. On the other hand, if the concentration of CO2 is very low, it induces the stomata opening even in the absence of light. Changes in the levels of CO2 content in the environs of plants are very well known. Due to active photosynthesis operating in the leaves, the concentration of CO2 decreases rapidly, but at nights, the CO2 concentration builds up because of respiration.
In recent years plant physiologists have demonstrated how the concentration of CO2 affects the cytoplasmic pH. Decrease in the concentration of CO2, the cellular pH increases, i.e. becomes more basic; the same is reversed if the concentration of CO2 is increased in the environs of leaves. The change(s) in the cellular pH has been found to effect or affect the activity of certain enzymes. In plants, increase in pH has been found to activate starch phosphorylase enzyme which activates the break down osmotically inactive starch into osmotically active glucose-P; while low pH can inactivate the starch phosphorylase but activates starch synthesizing enzymes. As a result, the concentrations of osmotically active molecules of guard cells change. This in turn brings about turgour movements of guard cells.
INTRA AND EXTRA CELLULAR MOISTURE CONTENT.
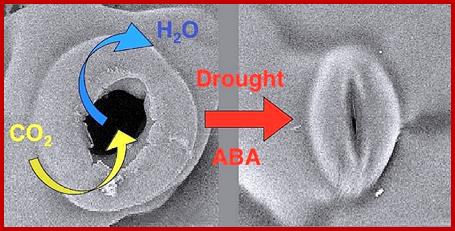
The moisture content of atmosphere is referred to as relative humidity. The RH of the atmosphere influences the rate of transpiration to a greater extent. But under extreme stress conditions like very low RH, in spite of favorable conditions like actinic light, low CO2 levels, etc. stomata close, this is because, the low RH acts as an unfavorable stress factor on guard cells, which immediately respond to such factors and induce closing. It has been found that under water stress conditions, whether it is due to a change in RH of the atmosphere or the non-availability of water, cells release abscissins that is a plant growth inhabiting hormone. Abscissin inturn brings about changes in the permeability of guard cells and induces the closing of stomatal opening similarly the non-availability of water in soil also induces closing of the stomata by the above said hormonal mechanism.
MECHANISM OF STOMATAL TRANSPIRATION:
GENERAL MECHANISM OF GUARD CELL MOVEMENTS
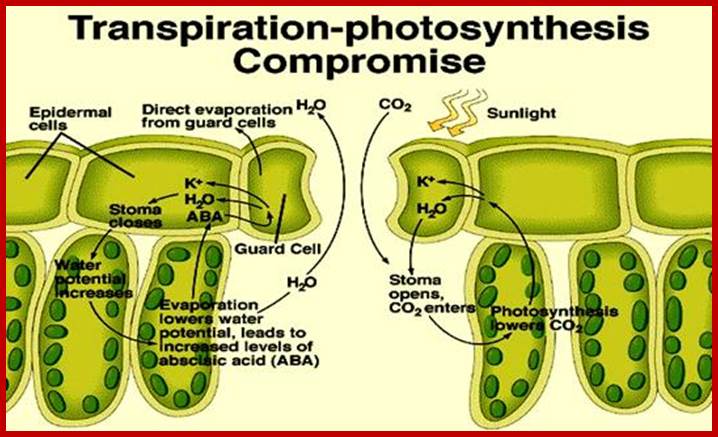
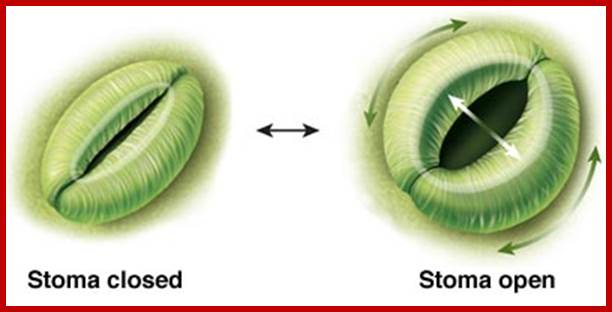
The most important single factor that is ultimately responsible for inducing the turgour movement is the change in the osmotic concentration or osmotic potential of guard cell. Once the osmotic pressure builds up in the guard cell, water from the neighboring cells moves into guard cells by aquaporins thus turgor pressure builds up within the cells. As the ends of the guard cells are tightly sealed, the cells can expand only in the lateral direction but not in length. As the cell wall of the guard cells towards subsidiary cells is thin, they are stretched laterally under increased turgour pressure. As a result, the anterior thick wall of the guard cell also buckles under tension; thus the stoma opens. The closing of stomata is almost reverse of the opening process, where the guard cell loose turgidity and come back to the normal position.
Most of the theories proposed from time to time have tried to explain the mechanism by which guard cells build up turgor pressure so guard cells can open and close the pores. Still, it is difficult to find any one single theory which explains all the operative forces leading to opening and closing of the stomata. Each one of the mechanisms simultaneously operates in inducing the opening and closing has been explained as a comprehensive scheme of events.
MECHANISM OF OPENING
With the onset of day, sunlight (blue and red light) triggers a series of reactions which have compounding and confluencing effects, which results in the turgour movement of guard cells to open the stoma (pore).
ACTIVATION OF STARCH HYDROLYSIS
The process of photosynthesis, which is triggered by the sunlight, with time fixes considerable amount of CO2 into carbohydrates. This causes a rapid fall in the concentration of CO2 in the immediate environs of the leaves including the inner spaces found within the mesophyll tissue. The decrease in the concentration of CO2 results in the increase of pH in the cellular cytoplasm. In guard cells, unlike other cells increase in the cellular pH activates enzyme similar to that of starch phosphorylase. The specific hydrolysing enzymes have an optimal pH; in this case it is 7.2 to 7.5. Such activated enzymes hydrolyze osmotically inactive polymers like amylose and amylopectins into glucose or glucose phosphates. The glucose phosphate is immediately dephosphorylated to glucose. It does not make much difference because both glucose and glucose phosphates are equally osmotically active. However, the increase in glucose concentration within the guard cell provides the motive force for the movement of water from subsidiary and other mesophyll cells into guard cells. As a result of the entry of water turgour pressure builds up and guard cells bulge with the outer cell wall is pushed and the inner thick wall is drawn inwards to open the pore. The presence and the activity of such starch hydrolysing enzymes in guard cells have been reported by Heath Zelith, Steward and others.
ACTIVATION OF ATPase POTASSIUM HYDROGEN PUMP
Light (blue and red-light wave lengths) besides inducing photosynthesis, activates cytokinin in guard cells which inturn activates ATPase dependent K/H pumps found in the plasma membranes of guard cells located towards subsidiary cells. Activated ATPase/H+ pumps, transport out H+ ions, which hyperpolarize guard cell membranes. Light also activates rectifying channels such as KAT1 and KAT2 and AKT1, transport of K+ ions from subsidiary cells
This causes hyperpolarization of the membrane, which also facilitates the movement of Cl -, Malate and Nitrate ions inwards. The activated pumps transport potassium ions, in massive quantities, from the subsidiary cells into the guard cells. Starch in apoplast gets hydrolyzed to sucrose and the same is pumped in.
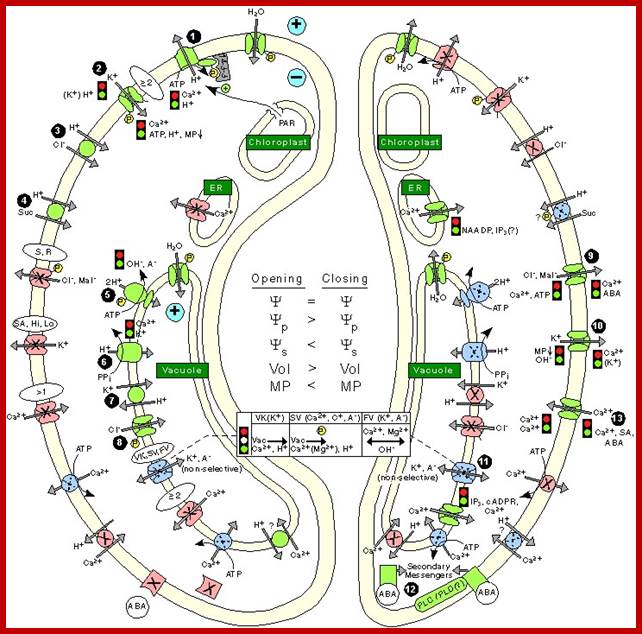
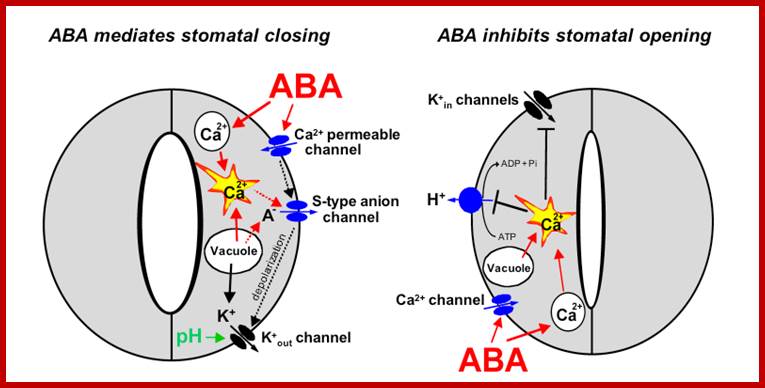
Stomatal opening is initiated by H+ extrusion1, which hyperpolarizes the PM and acidifies the apoplast. This effect increases the driving force of K+ uptake and activate voltage regulated K+-in channel Cl-; suc 4 may also be taken up. H+ pumping into the vacuole provides for K+ antiport and Cl_ uptake. GC (Guard Cell) solute increase leads to H2O uptake and therefore increases GC volume and aperture size. Stomatal closing is initiated by activation of A- channels, which depolarize the PM. This effect increases driving force for K+ efflux and activates voltage regulated K+-out channel. Several channels provide for solute release from the vacuole. Internal and external ABA receptors cause the release of Ca+ influx. ABA also induces stomatal closure by Ca+ independent means; http://bio.fsu.edu/
 Regulation of Ion channels, pumps and transporters localized in
the Plasma membrane of Guard cell during opening and closing of stomata: http://journal.frontiersin.org/
Regulation of Ion channels, pumps and transporters localized in
the Plasma membrane of Guard cell during opening and closing of stomata: http://journal.frontiersin.org/
This can activate starch found in apoplastic region to breakdown of starch into sugars, which are transported inwards. Guard cells which contain chloroplasts using sun light (BLUE and RED) fix CO2 and synthesize soluble sugars. All the buildup of solutes cause high osmotic pressure to pump in water by aquaporins (seven transmembrane proteins) and causes turgor pressure in guard cells which leads to swelling of guard cells where the thin outer cell wall extends into subsidiary cells outwards and inner tense thick wall is also pulled in, thus stomata open.
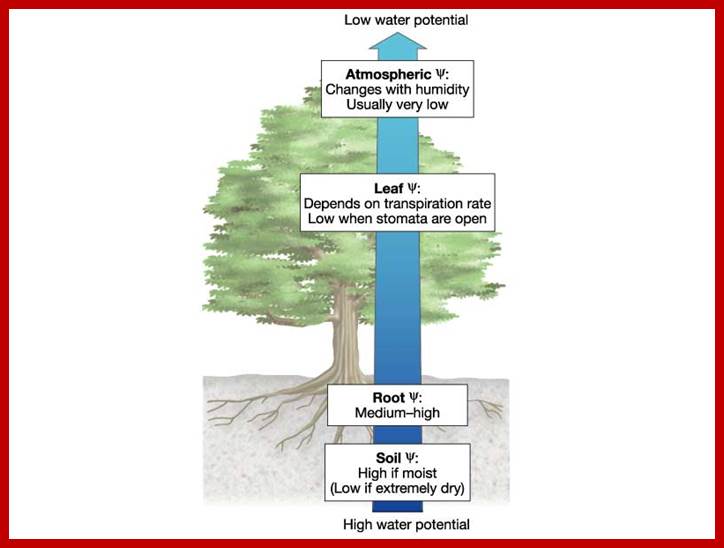
Water found in xylem elements diffuse into and fill the intercellular spaces in Apparent Free Spaces of mesophyll cells. Then water from AFS i.e. apparent free space and inter cellular spaces escape as water vapors into spaces found in the spongy and other parenchymatous tissue and finally into atmosphere, which provides the relative humidity of the atmospheric air present in the spaces is very low.
Mitochondrial Function; Triggers Stomatal Movement by Regulating Organic Acid Levels; http://www.plantcell.org;
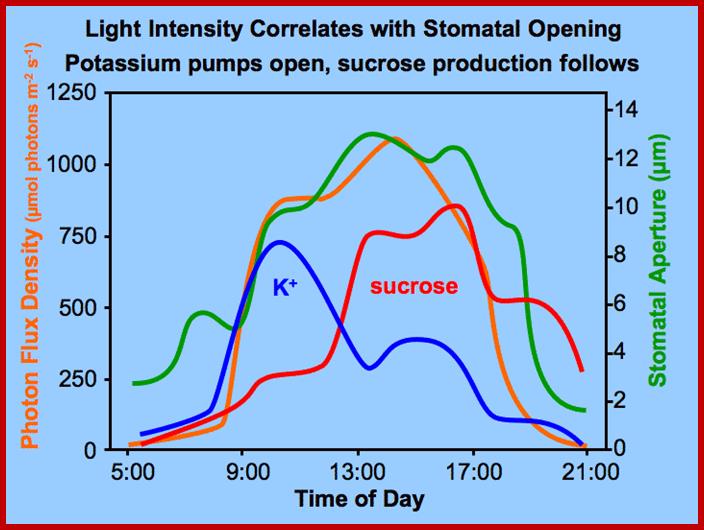
Light activated K+ pumps; https://plantstomata.wordpress.com/2015
http://plantphys.info/plant_physiology
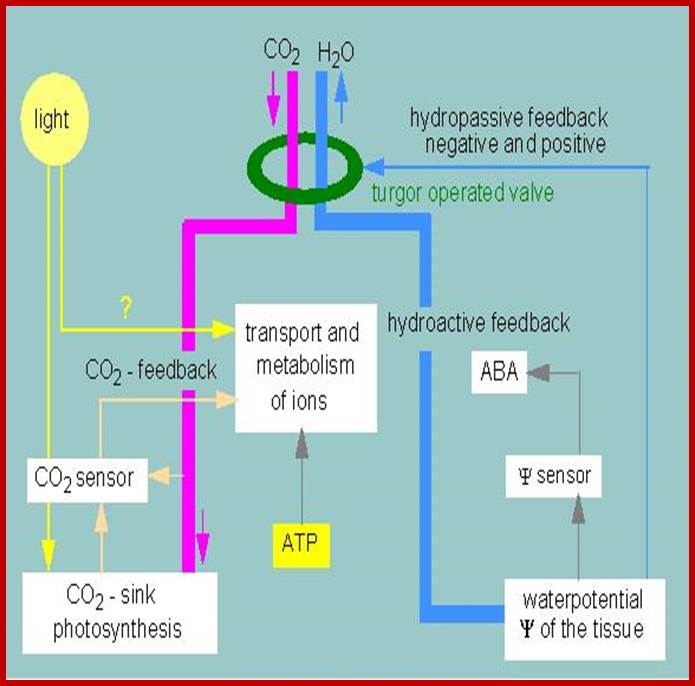
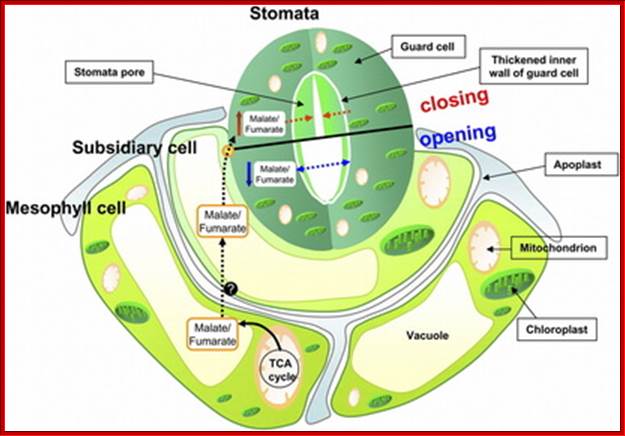
Controlling cycles regulating the movements of stomata. A guard cell is depicted schematically (according to K. RASCHKE,( 1975): www.s10.lite.msu.edu
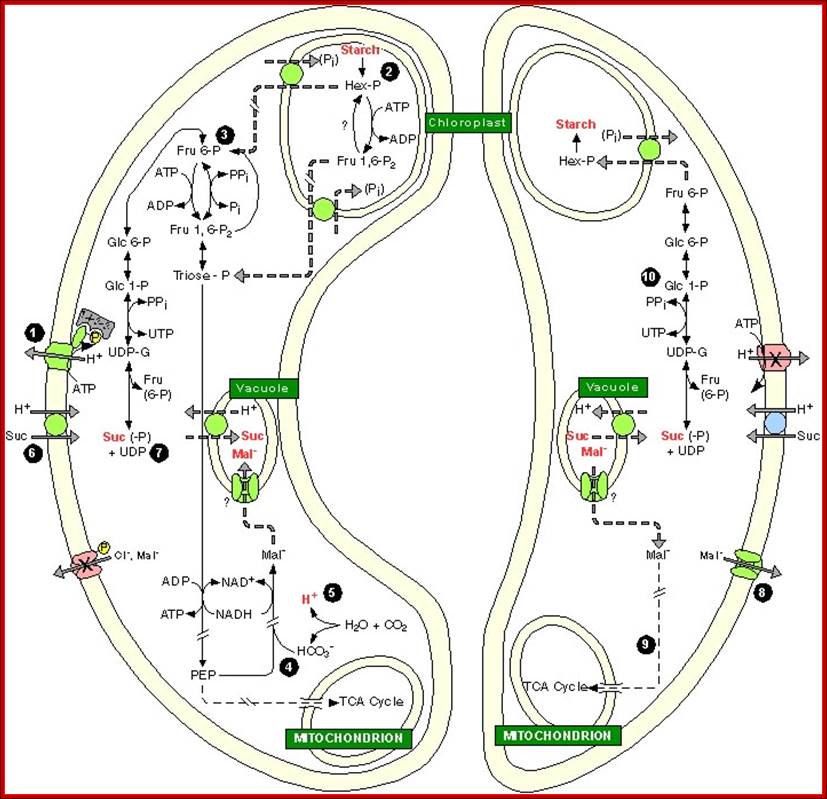
Opening- Left and Closing- Right; go-through carefully each of the steps; The role of Plastids and Mitochondria; http://bio.fsu.edu/ www.gopixpic.com; http://www.getdomainvids.com/
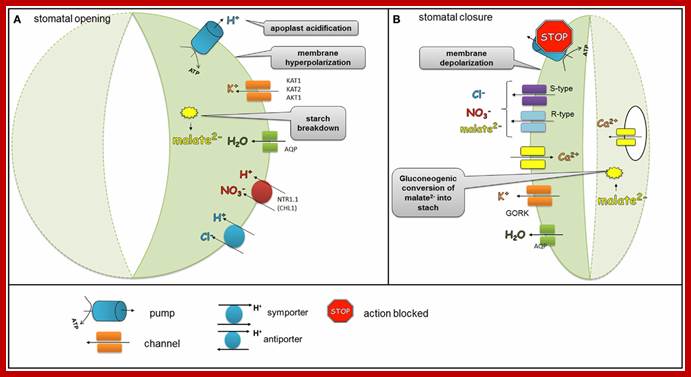
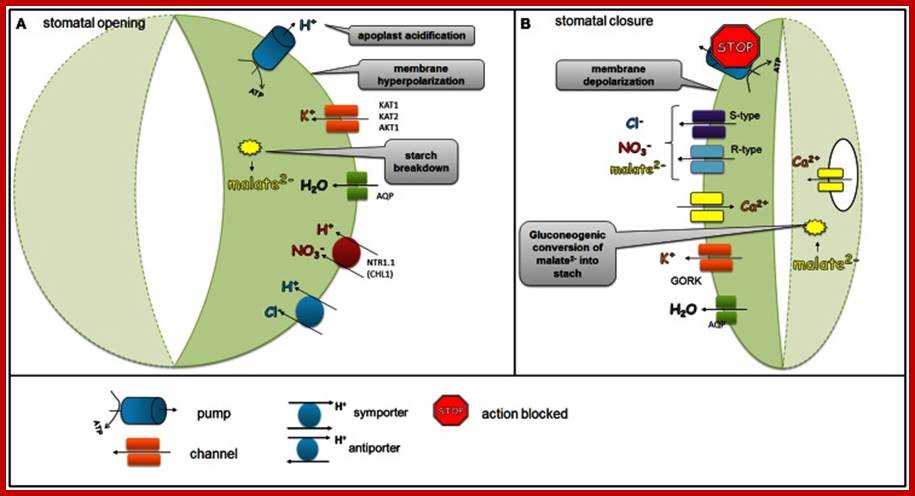
Regulation of ion channels, pumps, and transporters localized in the plasma membrane of the guard cells during stomatal opening and closure.
Blue and red lights in stomatal opening are confirmed. Stomatal opening is initiated by light which activates H+ ATPase pumps. During stomatal opening H+-ATPase pumps H+ from the guard cells and hyperpolarizes the membrane, which leads to the activation of K+ inward rectifying channels (KAT1, KAT2, AKT1). Anionic species such as malate2− from the breakdown of starch and transported NO−3 and Cl− ions contribute to the intracellular solute buildup that can mediate the import of sugars or can be used for the synthesis of sugars. Ions supplied into the guard cells together with water transported via aquaporins generate the turgor that is needed to keep stomata opened. As the plasma membrane is depolarized, S-type and R-type channels facilitate the efflux of malate2−, Cl−, and NO3-.
This is because, surrounding cytosolic H+ is low 10^7-10^8 M and weekly buffered (`10mM pH^-1). HCl symport provides H +source. In response hyperpolarization of membranes, the K+ ATPase pumps activated, now transport K+ ions in exchange of H+ ions. Along with it, starch/ sucrose found in apoplastic surfaces is degraded into sucrose. Starch is degraded to Hex-P which in cytosol and through glycolysis provides PEP. Glycolytic oxidation of aldehydes to acids releases one H+ to cytosol. PEP is gets carboxylated with HCO3, the synthesis of which releases a second H+.
Separately, PM hyperpolarization increases the driving force for sucrose uptake but control mechanism is not known, but now it is realized that apoplastic sucrose or starch found is metabolized. Some sucrose possibly formed from starch degradation. The same is pumped into guard cells. Guard cell symplastic (Suc) increase accounts for variable portion of the osmolyte requirements to open stomata, of which is in opposition to the GC apoplastic (Suc) increase that results from transpiration.
Photosynthesis in terms CO2 assimilation and synthesis of glucoses is not enough for stomatal cells to go into increased diffusion of water from subsidiary cells. But sucrose present in the Apoplastic region is activated and sucrose is imported into guard cell by a number of sucrose transporters. Import of sucrose, synthesis of glucose by guard cell by photosynthesis and import of large quantities of K+ ions all these causes cell osmotic imbalance, hence water diffuses from subsidiary cells in massive amounts into guard cells and subsidiary cells loose water and perhaps collapse. Aquaporins have the ability to pump in large amount of water in. Hence turgor pressure increases in guard cells and guard cells swell, in this process only the outer wall is stretched into subsidiary cells for they have lost most of their water. Enlargement is outwards to the central stomatal pore but not vertically for the ends of guard cells look like sealed and cushioned by epidermal layer of cells. In the process inner tensile thick wall is pulled inwards, this causes the opening of stomata. Opened stomata remain throughout the day till evening when photosynthesis slows down and CO2 concentration increases. At this point the prevailing light activates closing of the stomata.
During stomatal closure;
At onset of evening sunlight, H+-ATPases stop functioning and S-type and R-type anion channels are activated. At the same time, K+ outwardly rectifying channels such as GORK are activated through the depolarization of the membrane, which leads to the efflux of K+. As the plasma membrane is depolarized, S-type and R-type channels facilitate the efflux of malate2−, Cl−, and NO−3. The decreased level of malate2− is also caused by the gluconeogenic conversion of malate into starch. The elevation of the Ca2+ concentration due to transport of Ca2+ ions from vacuole across tonoplast membrane results in water movement from stomata into subsidiary cells or into intercellular spaces inside the mesophyll and also some into atmosphere, this results in loss of water.
The fate of sucrose during stomatal closure is unknown, but possibly it is converted to starch. (bio.fsu.edu). Because of the above factors mesophyll cells produce ABA and it moves into guard cells and binds to its specific receptor and the receptor activates, the process of closing of stomata.
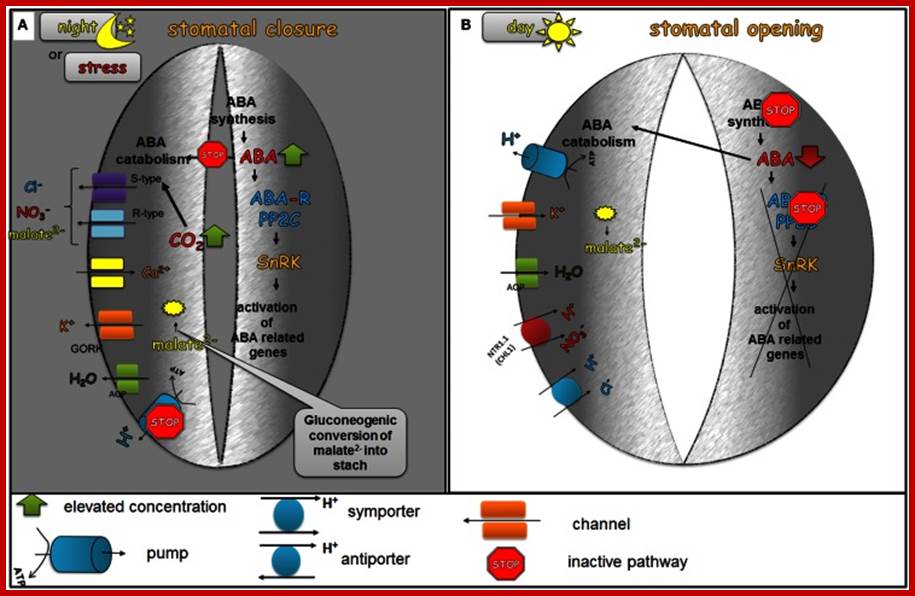
The role of ABA in the diurnal regulation of stomatal movements; In the dark phase of the day (A), ABA biosynthesis is favored and at the same time the catabolism of ABA is inhibited. As a result of these processes, elevated levels of ABA are present in the guard cells. ABA activates the efflux of Ca2+ from internal stores, the activation of S-type and R-type anion channels leading to the efflux of Cl−, malate2−, and NO−3, the activation of GORK channel, which leads to the efflux of K+ and consequently to the closing of stomatal pores. The decreased level of malate2− is also caused by the gluconeogenic conversion of malate into starch. In the dawn (B), the first light promotes ABA catabolism processes and the level of ABA biosynthesis decreases, which leads to a decreased concentration of active ABA in the guard cells. Low endogenous ABA levels no longer inhibit H+-ATPase (H+-pump), which is then able to extrude H+ from the guard cells. At the same time, the accumulation of water and ions, such as K+, Cl−, malate2− occurs in order to generate the turgor that is needed to keep stomata open. http://journal.frontiersin.org.
With light dims in the evening or any water stress causes ABA synthesis in mesophyll plastids and guard cell plastids and released; and it binds to its receptor, results in inhibition of H+-ATPase pump and K ions are effluxed by rectifying channels such as GORK. This is also accompanied with efflux of Cl-, NO3 and malate ions outward. It is at the same time Ca2+ ions are taken in and conduct Ca2+ ions from the central vacuole of the guard cell; any sucrose or hexose found is converted to inactive starch; these events cause reduced turgor pressure and water moves out of guard cells and where the thick tensile walls are pulled inwards, thus stomata close
Along with the above said events, that H2O2 generated activates Ca2+ channels in PM; Ca2+ ion enter into the guard cell, that leads to the exit of those which are pumped in; all this leads to reduction in turgidity of guard cell and cells restore to their opposition thus stomata close.
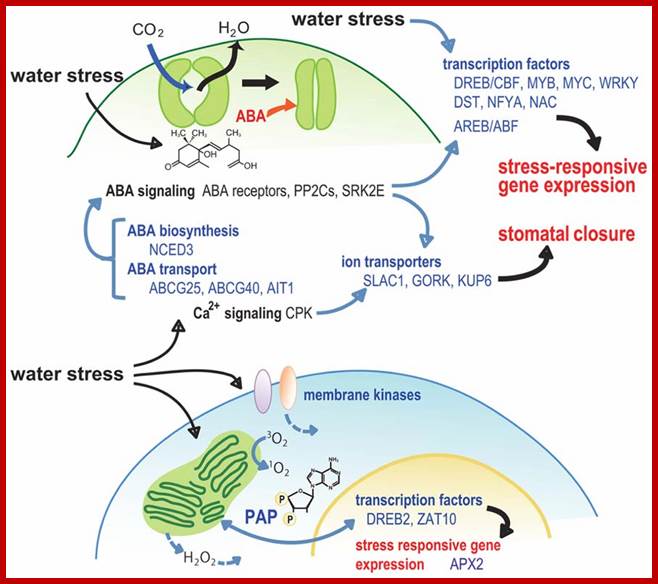
A model for the role of signaling factors in stomatal closure and retrograde signaling during water stress.; http://journal.frontiersin.org
But the activity and turgor movements of guard cells are further regulated by various factors like light, temperature, concentration of CO2 internal content of water, relative humidity of the atmosphere, etc.
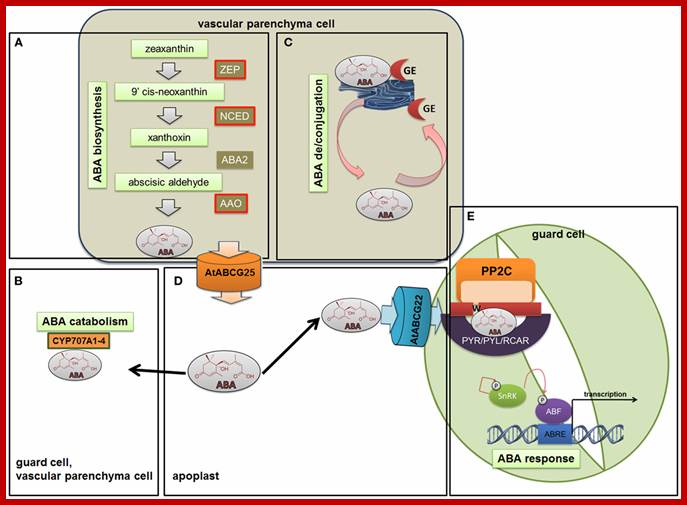
In response to environment ABA is synthesized; http://www.frontiersin.org/
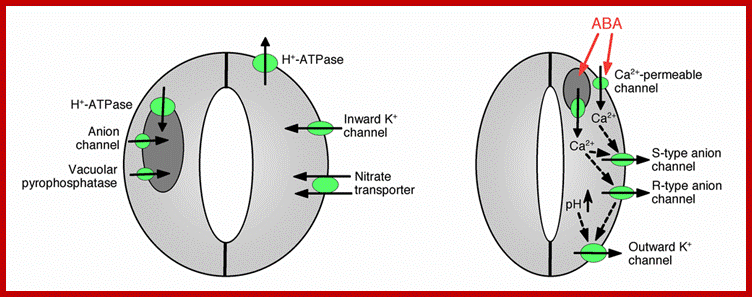
Ion channels and pumps regulate stomatal opening and closing; https://en.wikipedia.org.
K+ channels in Arabidopsis guard cells and their effectors. Changes in membrane potential lead to stomatal opening or closure, respectively. Membrane hyperpolarisation in response to H+-ATPase activity activates inward-rectifying K+ channels. Transcripts for KAT1, KAT2, AKT1, AKT2, and AtKC1 are detectable in guard cells (Szyroki et al., 2001). All these K+ channel subunits form hetero-tetrameric channels like KAT1-KAT2, AKT2-KAT2, and AKT1-AtKC1. The impact of AKT1 and Kin-AtKC1 on stomatal movement has not been investigated in detail. Acidification affects directly the currents through K+ channels in guard cells. Kin channels are activated upon extracellular acidification, while currents through Kweak channels decrease. Besides, the Kweak channel AKT2 is negatively affected by extracellular Ca2+. KAT1 currents are furthermore modulated by intracellular pH, ATP, and cGMP. ATP and cGMP have antagonistic effects. Moreover, stomatal K+ channels are affected by signals via signal transduction cascades. Effects of 14-3-3 proteins, Ca2+ and kinases have been reported. Membrane depolarization, on the other hand, caused by the inactivation of H+-ATPases and activation of anion channels activates the Kout channel GORK. GORK currents are positively influenced by extra- and intra-cellular alkalinisation. Furthermore, the current enhancing effect of H2O2 is under investigation. Both, stomatal opening and closure are affected by phytohormones. While Auxin evokes stomatal opening, ABA inhibits its opening but evokes closure of stomata. Abbreviations: pHac, acidification; pHba, alkalinisation.
Thereby, the osmotic concentration of K ions increases and that sets the chain of events like movement of water, increase in TP, and finally opening of stomata. In this process, subsidiary cells play a significant role in providing K+ ions for the guard cells. The energy required for K+ influx is supplied by noncyclic photophosphorylation activity of chloroplasts found within the guard cells. Potassium ions accumulation alone is not enough for osmotic concentration to change. This is supplied by Apoplastic movement sucrose into guard cells; all the causes opening of stomata. Phototropins like PHOt 1 and PHOt2 are found to be blue light receptors. http://journal.frontiersin.org/
http://journal.frontiersin.org/
Regulation of ion channels, pumps, and transporters localized in the plasma membrane of the guard cells during stomatal opening and closure:
During stomatal opening (A) H+-ATPase pumps H+ from the guard cells and hyperpolarizes the membrane, which leads to the activation of K+ inward rectifying channels (KAT1, KAT2, AKT1). Anionic species such as malate2− from the breakdown of starch and transported NO−3 and Cl− ions contribute to the intracellular solute buildup that can mediate the import of sugars or can be used for the synthesis of sugars. Ions supplied into the guard cells together with water transported via aquaporins generate the turgor that is needed to keep stomata opened. During stomatal closure (B), H+-ATPase is inhibited and S-type and R-type anion channels are activated. As the plasma membrane is depolarized, S-type and R-type channels facilitate the efflux of malate2−, Cl−, and NO−3. At the same time, K+ outwardly rectifying channels such as GORK are activated through the depolarization of the membrane, which leads to the efflux of K+. The decreased level of malate2− is also caused by the gluconeogenic conversion of malate into starch. The elevation of the Ca2+ concentration as a result of the release of Ca2+- via channels situated in both the plasma membrane and in the tonoplast is another event that accompanies stomatal closure.
The role of ABA in the diurnal regulation of stomatal movements;
In the dark phase of the day (A), ABA biosynthesis is favored and at the same time the catabolism of ABA is inhibited. As a result of these processes, elevated levels of ABA are present in the guard cells. ABA activates the efflux of Ca2+ from internal stores, the activation of S-type and R-type anion channels leading to the efflux of Cl−, malate2−, and NO−3, the activation of GORK channel, which leads to the efflux of K+ and consequently to the closing of stomatal pores. The decreased level of malate2− is also caused by the gluconeogenic conversion of malate into starch. In the dawn (B), the first light promotes ABA catabolism processes and the level of ABA biosynthesis decreases, which leads to a decreased concentration of active ABA in the guard cells. Low endogenous ABA levels no longer inhibit H+-ATPase (H+-pump), which is then able to extrude H+ from the guard cells. At the same time, the accumulation of water and ions, such as K+, Cl−, malate2− occurs in order to generate the turgor that is needed to keep stomata open.
ABA triggers cytosolic calcium ([Ca2+]cyt) increases (McAinsh et al., 1990; Fig. 1, left panel). [Ca2+]cyt elevations activate two different types of anion channels: Slow-activating sustained (S-type; Schroeder and Hagiwara, 1989) and rapid transient (R-type; Heidrich et al., 1990) anion channels. Both mediate anion release from guard cells, causing depolarization (Fig. 1, left panel). This change in membrane potential deactivates inward-rectifying K+(K+in) channels and activates outward-rectifying K+ (K+out) channels (Schroeder et al., 1987), resulting in K+ efflux from guard cells (Fig. 1, left panel). In addition, ABA causes an alkalization of the guard cell cytosol (Blatt and Armstrong, 1993) which directly enhances K+out channel activity (Blatt and Armstrong, 1993; Ilan et al., 1994;Miedema and Assmann, 1996) and down-regulates the transient R-type anion channels (Schulz-Lessdorf et al., 1996). The sustained efflux of both anions and K+ from guard cells via anion and K+out channels contribute to loss of guard cell turgor, which leads to stomatal closing.
As vacuoles can take up over 90% of the guard
cell’s volume, over 90% of the ions exported from the cell during stomatal
closing must first be transported from vacuoles into the cytosol (MacRobbie, 1998; MacRobbie, 1995). [Ca2+]cyt elevation activates vacuolar K+ (VK) channels proposed to provide a
pathway for Ca2+-induced K+ release from the vacuole (Ward and Schroeder, 1994). At resting [Ca2+]cyt,
K+ efflux from guard
cell vacuoles can be mediated by fast vacuolar (FV) channels, allowing for
versatile vacuolar K+ efflux
pathways (Allen and Sanders, 1996). The pathways for anion release
from vacuoles remain elusive.
Stomatal opening is driven by plasma membrane
proton-extruding H+-ATPases. H+-ATPases can drive K+uptake
via K+in channels (Fig. 1, right panel; Kwak et al., 2001). Cytosolic Ca2+ elevations in guard cells
down-regulate both K+in channels
(Schroeder and Hagiwara, 1989) and plasma membrane H+-ATPases
(Kinoshita et al., 1995), providing a mechanistic basis for
ABA and Ca2+ inhibition
of K+ uptake during
stomatal opening .
(Fig.
1, right panel).
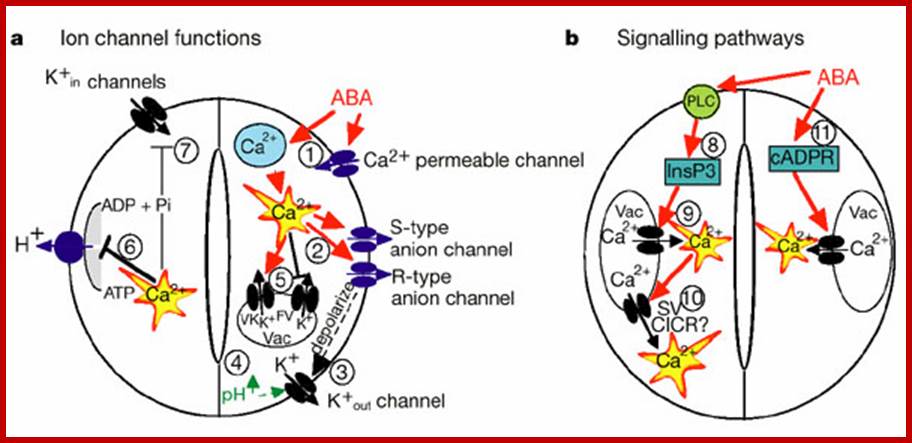
Components and pathways of guard cell ABA signaling; Guard cell abscisic acid signalling and engineering drought hardiness in plants; Schroeder, June M. Kwak and Gethyn J. Allen; Nature 410, 327-330(15 March 2001); http://www.nature.com/
a, ABA is detected by as yet unidentified receptors (right guard cell) and induces cytosolic Ca2+ elevations (1) through extracellular Ca2+ influx and release from intracellular stores (for reviews see refs 2, 8, 9). (2) [Ca2+] elevations activate two types of anion channel that mediate anion release from guard cells, slow-activating sustained (S-type) or rapid transient (R-type) anion channels3, 8. (3) Anion efflux causes depolarization, which activates outward-rectifying K+ (K+out) channels and results in K+ efflux from guard cells2, 3. (4) ABA causes an alkalization of the guard cell cytosol, which enhances K+out channel activity44. Overall, the long-term efflux of both anions and K+ from guard cells contributes to the loss of guard cell turgor, leading to stomatal closing3. Over 90% of the ions released from the cell during stomatal closing must be first released from vacuoles into the cytosol. (5) At the vacuole, [Ca2+]cyt elevation activates vacuolar K+ (VK) channels, which are thought to mediate Ca2+-induced K+ release from the vacuole8. In addition, fast vacuolar (FV) channels can mediate K+ efflux from guard cell vacuoles at resting [Ca2+]. ABA also inhibits ion uptake, which is required for stomatal opening (left guard cell). (6) [Ca2+]cyt elevations inhibit the electrogenic plasma membrane proton-extruding H+-ATPases46 and K+ uptake (K+in) channels2, 3, 44 (7). Initiation of ion efflux (1–5, right guard cell) and inhibition of stomatal opening processes (6, 7, left guard cell) provide a mechanistic basis for ABA-induced stomatal closing.
b, Ca2+-releasing second messengers that mediate ABA-induced stomatal closing. Second messengers have been implicated in ABA signalling in guard cells, including InsP3 and cADPR. (8) Biochemical evidence indicates that ABA stimulates InsP3 production in guard cells2. (9) InsP3 can release Ca2+ from intracellular stores, inhibit K+inchannels and cause stomatal closure9, 13, 22, 44. (10) [Ca2+]cyt elevations may be amplified by a Ca2+-induced Ca2+release (CICR) mechanism from the vacuole through activation of the Ca2+-dependent slow vacuolar (SV) channel8,45. Data questioning this SV model for CICR47 and recent other data supporting it48 indicate the potential for molecular genetic analyses. (11) ABA stimulates the production of cADPR in plant cells49, which also increases [Ca2+]cyt in guard cells20, 45 and promotes stomatal closing.
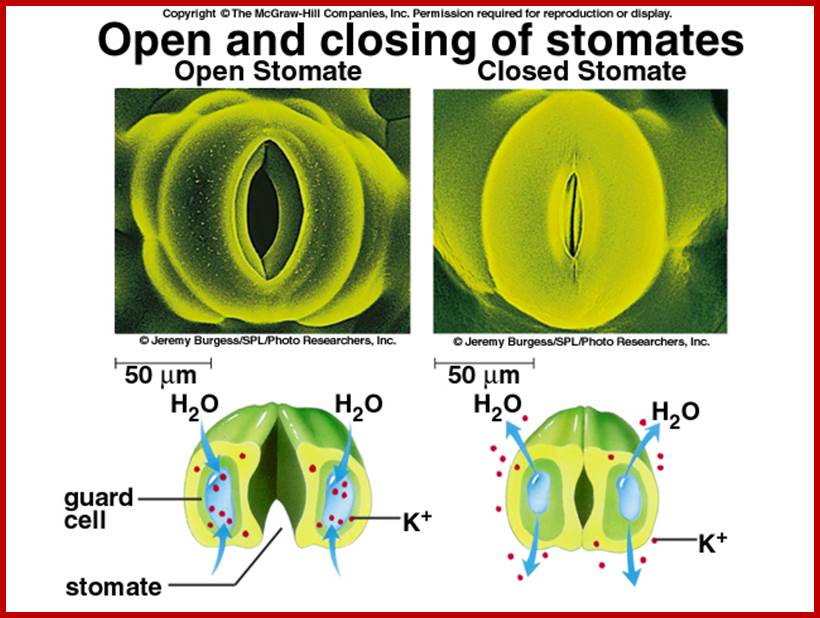
Basic mechanism; www.mhhe.com
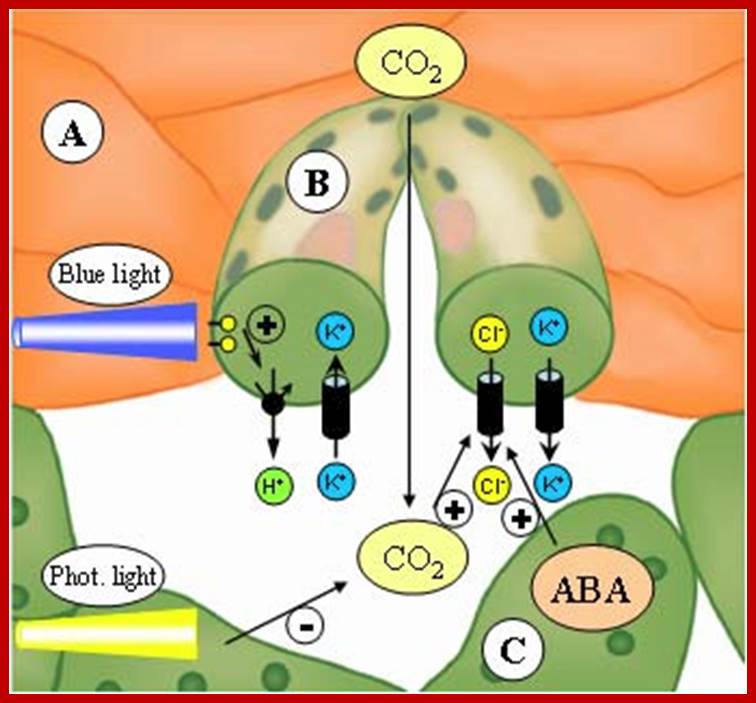
Signal transduction mechanisms in guard cells integrate a multitude of different stimuli to modulate stomatal aperture. Stomata open in response to light. In response to drought stress, plants synthesize the hormone abscisic acid (ABA) which triggers closing of stomatal pores. Guard cells have become a well-developed system for dissecting early signal transduction mechanisms in plants and for elucidating how individual signaling mechanisms can interact within a network in a single cell. Previous reviews have described pharmacological modulators that affect guard cell signal transduction. Here we focus on mechanisms for which genes and mutations have been characterized, including signaling components for which there is substantial biochemical evidence such as ion channels which represent targets of early signal transduction mechanisms. Guard cell signaling pathways are illustrated as graphically linked models: Pascal Mäser et al.www.labs.biology.ucsd.edu
A peep through anion channles ; Sébastien Thomine, & Hélène Barbier-Brygoo
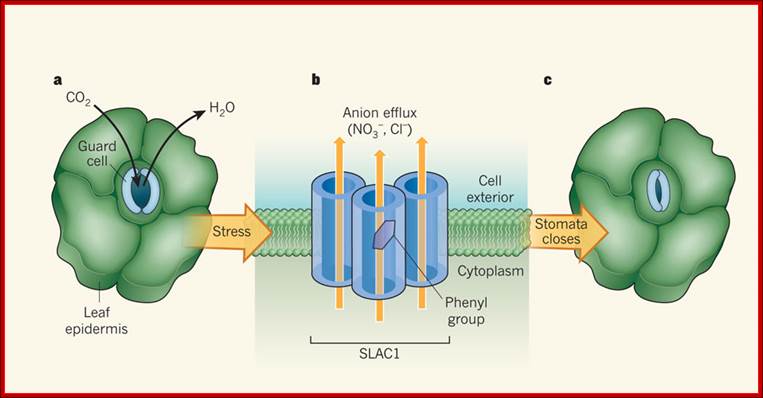
Role of SLAC1 channels in stomatal closure:
a, In the leaf epidermis of terrestrial plants, pores known as stomata regulate CO2 uptake and the loss of water vapor. The pores are formed from two kidney-shaped guard cells. b, The SLAC1 channel in the cell membrane of guard cells is responsible for nitrate (NO3−) and chloride (Cl−) efflux from the cells, a process that is triggered by stressful stimuli such as drought. Chen et al. report the crystal structure of the bacterial protein TehA, the amino-acid sequence of which is related to SLAC1. Their model of SLAC1, based on the TehA structure, indicates that three subunits of the protein each form a pore and associate into 'triple-barrel' structures in the membrane. When closed, the pore channel of each subunit is occluded by the phenyl group of an amino-acid residue, forming the basis of a previously unknown gating mechanism common to plant and bacterial channels from the SLAC family. Here, a phenyl group is shown in an 'open' orientation; the phenyl groups in the other two subunits are not shown. c, the coordinated efflux of nitrate and chloride ions triggers further efflux of potassium ions and water from guard cells (not shown), resulting in stomatal closure. www.nature.com
With the sun setting, the process of photosynthesis stops; as a result three set of reactions which are initiated during the opening of stomata also stops. However, respiratory process continues, with the result the concentration of CO2 gradually increases and oxygen level decreases. With the increase in the levels of CO2, the pH of the guard cells decreases. Hence, starch hydrolyzing enzymes become inactive, but the same pH activates the enzymes responsible for the synthesis of osmotically inactive starch by utilizing the glucoses available in the guard cells. Hence, the osmotic pressure of guard cells decreases and water starts moving out into subsidiary cells. While subsidiary cells are enlarging guard cells lose their cellular TP and regain the normal shape thus the stomata close. Similarly, increase in carbon dioxide and decrease in oxygen inhibits glycollate/malate metabolism; which further affects the osmoticum of guard cells. Finally, it leads to closing of the stomata.
Furthermore, the change in the PH of guard cells due to increase in CO2 during night also induces the release of abscisic acid from the chloroplasts (ABA is synthesized in plastids). Abscissin has a reverse effect on the ATPase pump, where the influx of K ions is stopped. Instead, the efflux of K ions is induced. Thus the guard cells loose osmotic concentration, which inturn induces the closing of the stomata. In recent years the effect of ABA on turgour movement of cells has gained credibility, because of many experimental evidences. The abscisic acid is now certainly known to induce the closing of stomata. In fact, the mid day closure of stomata due to water stress, is attributed to ABA is effect, because under water stress condition, the synthesis and release of ABA increases significantly. All in all the above mentioned pathways have a compounding effect on the DPD of the guard cells, which ultimately leads to the closure of stomata.
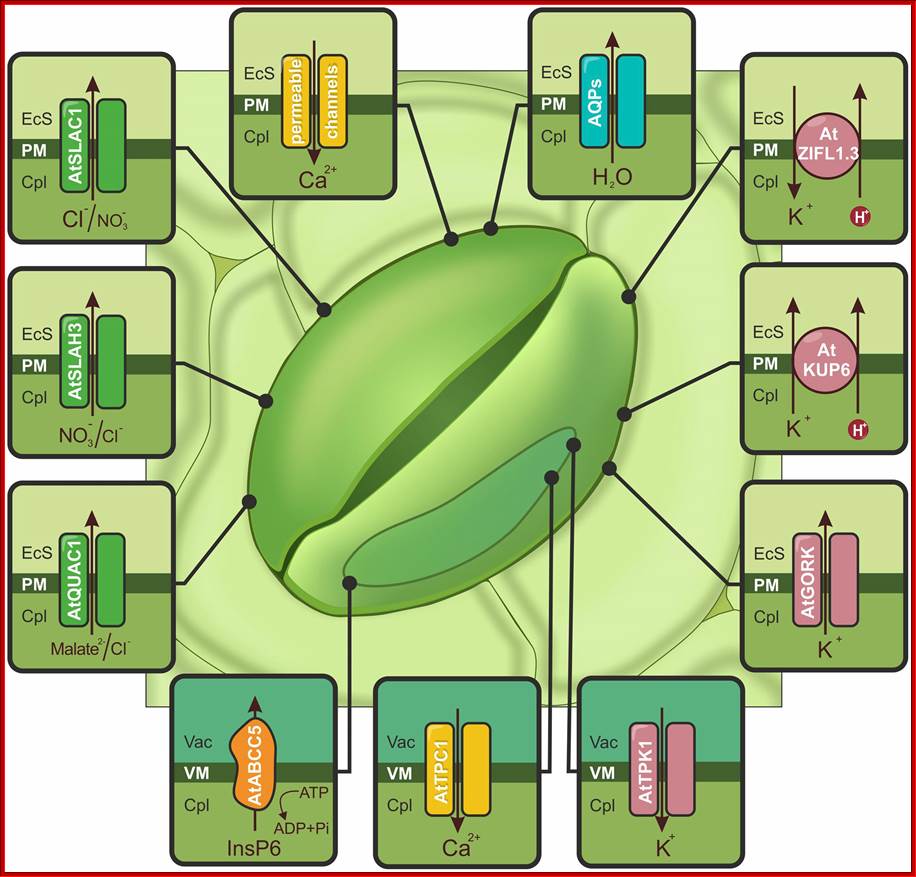
Schematic illustration of ion channels, aquaporins (AQPs) and transporters activated by drought or ABA and controlling stomatal closure: The accumulation of cytoplasmic Ca2+ ([Ca2+]cyt), is possible due to Ca2+-permeable channels (yellow) localized in the PM (e.g., AtCNGC5, AtCNGC6, AtGLR3.1) and the tonoplast (AtTPC1). Increasing the amount of calcium activates S-type (AtSLAC1 and AtSLAH3) and R-type (AtQUAC1) channels, which are indicated in green. AtABCC5/MRP5 (orange), localized to the vacuolar membrane (VM), is a regulator of Ca2+-permeable and S-type channels. The actions of the S- and R-type channels induce membrane depolarization and activate K+ flow through AtGORK and AtKUP6 (pink) from guard cells. The release of K+ from the vacuole is mediated by the AtTPK1 channel (pink). Additionally, stomatal closure is regulated by the action of the AtZIFL1.3 isoform, indicated in pink. AQPs are responsible for the outflow of water (e.g., VfPIP1), shown in blue. EcS, extracellular space; Cpl, cytoplasm; Vac, vacuole; http://journal.frontiersin.org/
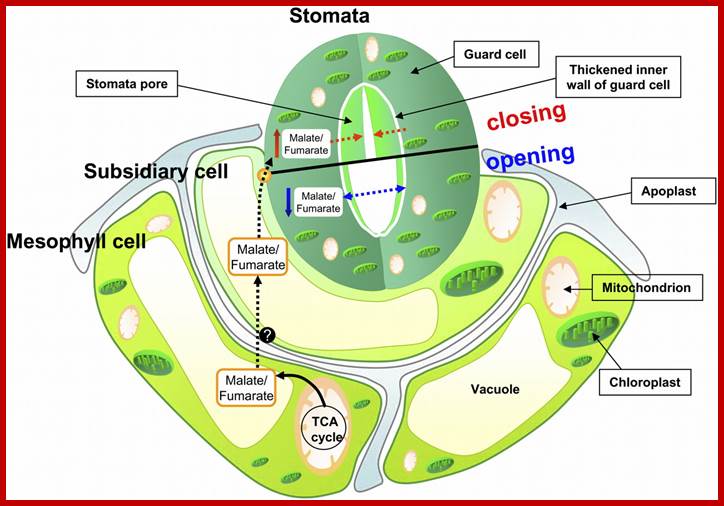
ACTIVATION OF GLYCOLLATE / MALATE METABOLISM:
Photosynthetic activity in green leaves have two important effects; first it decreases the concentration of CO2 and the second it increases the intracellular concentration of oxygen; the latter triggers the glycollate metabolism. In the presence of higher concentration of oxygen, RUBP carboxylase found in chloroplasts acts as RUBP oxygenease. This enzyme oxidizes the RUBP to phosphoglycerate and glycollate phosphate. Synthesis and accumulation of glycollate along with non-cyclic ATP formation in guard cells has been observed. Zelith and Walker have further shown that glycollate is very effective in the opening of stomata. To top it, the chloroplasts found in guard cells have been found to synthesize malate by PEP carboxylase activity. The glycollate and malate metabolism not only provides energy and hydrogen ions for ATPase pumps, but they also contribute in building up of osmotic concentration, which initiates the events like entry of water, increase in ATP and finally the opening of the stomata.
In all the above said reactions light triggers various events where each pathway leads to the accumulation of one or other chemical components thus cause a DPD gradient between guard cells and subsidiary cells. Even if one of the above mentioned three pathways is not operating, stomata can still open with other mechanisms. With all the pathways operating the total effect is confluencing and rapid. As subsidiary cells provide K+ ions and water in large quantities for the guard cells, they play an important role in opening and closing of the stomata.
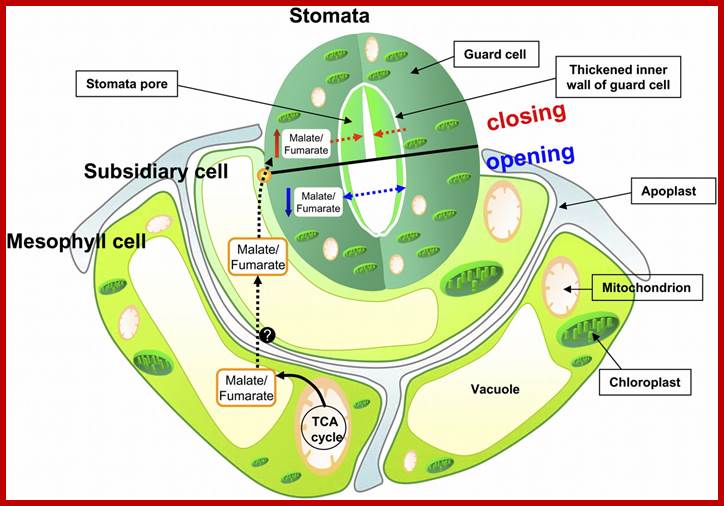
Mitochondrial Function; Triggers Stomatal Movement by Regulating Organic Acid Levels. http://www.plantcell.org;
 http://www.ncbi.nlm.nih.gov/
http://www.ncbi.nlm.nih.gov/
Genetics of stomatal operation:
Quantitative trait analysis (QTL), instead of mutational analysis, has shown a number of QTLs affecting transpiration efficiency in Arabidopsis. Now it is to find out what these loci encode in regulating stomatal opening and closing. One QTL analysis one genetic locus, ARECTA which encodes a Leu- rich repeat sequences containing receptor like kinase as a genetic regulator of transpirational efficiency (Masle et al ,2005).
When plants are drought stressed, the plant hormone ABA accumulates in shoot where it inhibits opening but promotes stomatal closure, resulting in reduced water evaporation.
In addition to the above factors mentioned including signals gene expression changes have been implicated in regulating stomatal opening and closing and the stomatal loss of water. Two R2R3-MYB domain TF AtMYB60 and AtMYB61 T-DNA insertional mutants show reduced sensitivity towards light – induced stomatal opening, reduced water loss when draught stress was measured by water the relative water content of the rosette leaves; there appears that atm yb60 AND atm yb61 have functional specificity in diurnal regulation of stomatal aperture and transpirational water loss.
FACTORS THAT CONTROL TRANSPIRATION :
Whatever factors that regulate the movement of guard cells also control the rate of transpiration. Among them light, temperature, RH of the atmosphere, soil water, air currents, stomatal index, etc. are the major factors that control the rate of transpiration.
LIGHT:
Sunlight is an important source of energy for all living organisms. During photosynthesis plants capture, convert and conserve the solar energy in the form of chemical energy. In fact, light also triggers a series of multipronged reactions within the guard cells, which ultimately leads to the opening of the stomata, thus transpiration is favored in day time, but not during nights.
TEMPERATURE:
Most of the plants, both in tropical and temperate regions grow luxuriantly at 25 0C to 30 0C. Because of this 25-30 0C is the most favored temperature for maximum enzymatic activities. So the same temperature is also effective in opening of the stomata, hence the loss of water by transpiration. Low temperature, as prevailed in certain temperate regions, plants do not loose any water because stomata remains closed. But increased temperature, though it felicitates the opening of stomata, has a profound effect on the relative humidity of the atmosphere, which may have a compounding effect.
RELATIVE HUMIDITY ATMOSPHERE:
The relative moisture content of the atmosphere is called relative humidity which greatly varies, because of various factors like, season of the year, altitude, location, temperature, etc. For example, in summer season, because of high temperature, the RH of the atmosphere is low and the rate of transpiration is always high. The reverse is the case on a rainy day during rainy season. Plants growing either in temperate climate or tropical climates, at very high altitudes, because of extreme low temperatures stomata remain closed and they don’t loose any water. So the on the contrary, if the temperature is high the RH will below so the effect of temperature on relative humidity is very significant. So also it affects the opening and closing of the stomata.
Another significant effect of RH on transpiration is the rate of transpiration and the magnitude of water loss. At 50% RH, the DPD of the atmosphere is about 1000 bars, while the DPD of the leaf cells is just about 40 bars or less than that. A situation like this creates such a steep gradient, water is virtually pulled out of the plants into the atmosphere hence the transpiration is greatly facilitated. The rapidity with which plants loose water is more or less controlled by the relative humidity of the atmosphere, provided that the other factors are favourable for the stomata to remain open or closed.
SOIL WATER:
Water found in the soil is absorbed by the roots and the same is transported through the stem to the aerial regions of the plant body. And finally most of the water is lost by the way of transpiration. Thus the water is continuously depleted from the soil to atmosphere through the plant structures. If water is available in the soil and conditions are favourable for transpiration, plants go on depleting the water from soil eternally. But the availability of water in the soil, at times, acts as a deterrent factor in transpiration. Adequate supply of water to transpiring plant is not found, the stomata close, because of water stress. This state continues till the root system is replenished with sufficient water. Thus the soil water content plays an important role in the loss of water.
AIR CURRENTS:
The velocity of wind over the surface of leaves contributes a greater impact on the water potential gradient between the surrounding atmosphere and the plant surfaces. In still air, depending upon the DPD gradient, plants may transpire water rapidly or slowly. But with high winds velocity water is displaced from the surface of leaves rapidly, which results is greater loss of water, however under such conditions of water stress, stomata close and inhibit transpiration.
TRANSPIRING STRUCTURES:
Leaves are the most important structures responsible for transpiration. The number of stomata and distribution of stomata provide the potential surface areas for transpiration. Xerophytes contain reduced or modified leaves, where transpiration is almost nil. On the contrary, a mesophyte has a greater number of stomata, which results in the greater loss of water per unit area per a unit of time. While plants like Potomegaton, though living and leading a submerged habitat in water, they don’t loose any water, for they have non functional stomata. But under normal conditions, if the surface area is provided for transpiration, plants loose greater amount of water and vice versa. The structural adaptation of the transpiration is mostly dependent on the genetic potentiality as well as the plasticity with which they adapt to the environment.
IMPORTANCE OF TRANSPIRATION:
Evil effects:
The process of transpiration has been damned as an unavoidable evil. It is more or less true; because the vagaries of rain, which determines the supply of water to plants. Whether water is available or not, plants go on loosing water till they wilt temporarily or permanently; probably it may lead to death if the conditions are hostile. Insufficient supply of water, due to the rapid depletion of water in the soil causes a greater stress on the metabolism of plant cells and on its growth and development, then it prevents the absorption and transportation of minerals to different regions of the plant. This also has a greater bearing on the food production of the world.
The above said observation has necessitated the research workers to find out some substances which prevent transpiration which are fashionably called anti transpirants. Substances like phenyl mercuric acetate, hydroxydeconol, etc., on spraying, they are found to form very thin films over the surfaces of leaves and prevent the loss of water. But some of these substances have also been found to be deterrents, in the sense; they prevent the entry of CO2 into leaves, which has deteriorating effect on photosynthetic yield. Search for genetic variants where the most important crop, plants like cereals, pulses, which provide the food for the entire human population on this planet, could or should possess no stomata at all or have non functional vestigial stomata, thus farmers can grow their crops and harvest with one or two rains without endangering them to drought.
OTHER EFFECTS:
Rapid transpiration brings in an overall effect on recirculation of water within the cells. This also leads to the development of mechanical tissue in the neighborhood of translocation structure, probably to give strength to such structures. The rapid transpiration depletes the water from the soil, which probably provides a motive force for the extensive growth of roots in search of water.
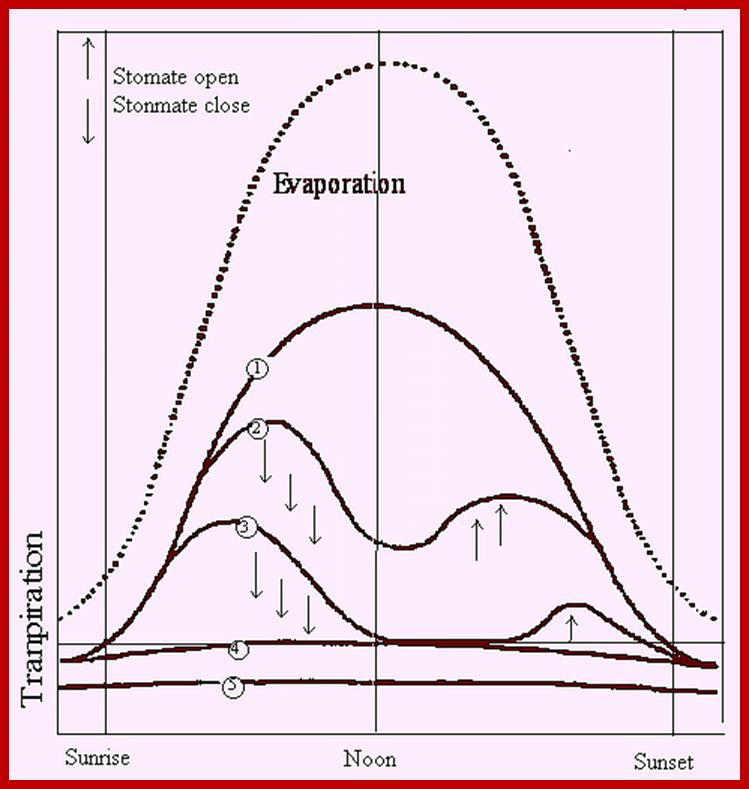
Diurnal rhythm of opening and closing of stomata; www.images.1233.tw