Fluid transport 2
TRANSLOCATION OF ORGANIC SOLUTES
Young and growing structures of the plant body like shoot apex, root apex, leaf primordial, floral buds, axially buds, etc. require vitamin, hormones and adequate energy source for their active metabolism and sustained growth. Similarly, growing fruits and other storage organs require reserve food materials for their normal development.

Movement of minerals, water and organic solutes via xylem and phloem elements; www.journal.frontirsin.org; http://aobblog.com/
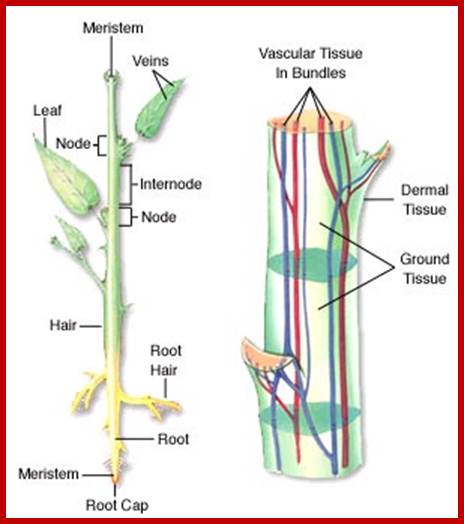
Vascular bundles one Xylem that transports water and minerals and another sieve tubes that transports organic solutes.; http://www.mhhe.com/

Typical plant cell; http://www.clt.astate.edu/


Organic solutes move upwards and own ward from the leaves; www.scienceaid.co.uk
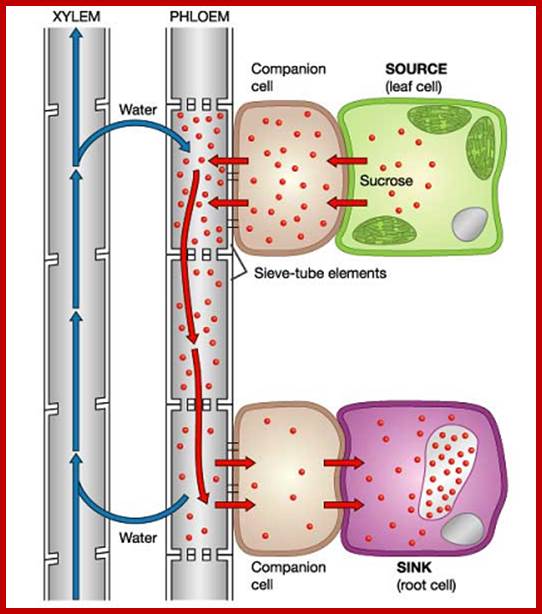
The said organic substances are mostly synthesized or made available in the photosynthetic organs like leaves, phyllocades or cladodes, from where, i.e., supply end, organic substances are transported to structures where it is needed. This end is called as the sink and the supply end is called the source. Such a process is called translocation of organic solutes.

www.gopixpic.com’wonderwhizkids.

Schematic drawing of sieve tube structure in Arabidopsis thaliana. C = chloroplast, Cl = clamp proteins, ER = endoplasmic reticulum, EV = electron dense vesicles, GM = ground matrix, M = mitochondrium, N = nucleus, P = plastid, SR = SEOR1 filaments, V = vacuole. From Froelich et al. 2011. Phloem ultrastructure and pressure flow: SEOR protein agglomerations do not affect translocation. Plant Cell . Plant Cell doi/10.1105/tpc.111.093179 Copyright American Society of Plant Biologists; http://public.wsu.edu/
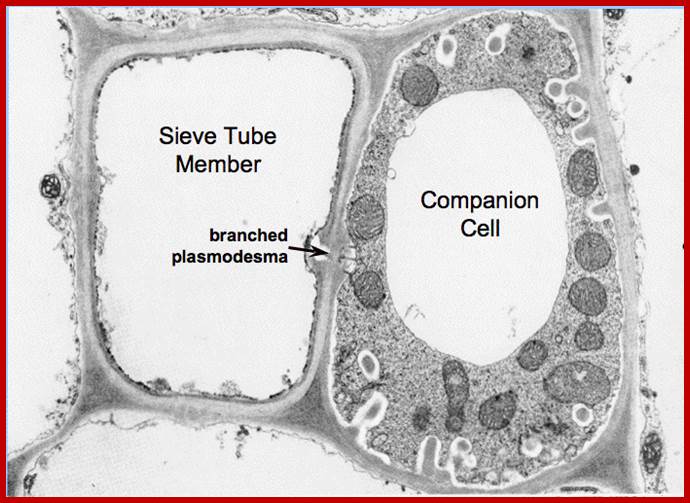
Companion cells in Plants; www.study.com

1 = companion cells; 2 = sieve tube content; 3 = parenchyma cell; 4 = sieve plate (oblique view); 5 = one sieve element; 6 = sieve plate (side view); 7 = plasmalemma of the sieve tube; 8 = original site of moving sieve tube sap, but possibly P-protein due to artifact of fixation; 9 = plastids; 10 = phloem parenchyma cell

0.1-15um,http://public.wsu.edu/
Sieve tubes are living cells which contain cytoplasm but do not have nucleus. So, its function is supported by companion cells. Each sieve tube has one or two companion cells bound to it for both companion cell and sieve tube cell are derived from the same progenitor cell. Companion cell can undergo one or two cell divisions; thus, each sieve tube cell is associated with more than one or two companion cells. Sieve tube cells are also associated with parenchyma cells and aluminous cells in gymnosperms which lack starch. Sieve tube cells do contain at earlier stages the nucleus gets degraded but, retain sieve cell mRNAs, ribosomes, miRNAs 165/166, ER and mitochondria and modified plastids at the peripheral cytoplasmic area. Sieve tube cells are full of organic compounds especially photosynthates from leaves.
Companion cells and sieve tube cells are connected by a large number of plasmodesmata. Sieve cells also contain Phloem specific elements called P-proteins in large amounts. Some companion cells have ingrowth that facilitates apoplastic transport of sucrose into symplast. Many closely associated phloem parenchymatous cells with development of inward protrusions develop into transfer cells, where they are involved in transport of solutes into sieve cells.
Phloem sieve cells form a kind of network in transporting photosynthates and signal molecules. The phloem differentiation and development start, with meristems and leads to proto-phloem leads to the development of fully formed sieve elements. During development key transcription factors regulate phloem development. Neighboring cells communicate with developing sieve elements apoplastically as well as symplastic pathways.
Phloem is involved in transport of several phytohormones and the same distributed; auxin, CKs, Abscisic acid, and Gibberellins, Jasmonic acids, salicylic acids, and/or their derivatives are also proposed as components of the phloem sap in association with defense signaling. IAA and other IAA derivatives and cytokinins have been identified from phloem sap.

Plasmodesmata. Plasma membrane-lined pores called plasmodesmata (PD) penetrate cell walls (CW) of neighboring cells. Compressed endoplasmic reticulum (ER), called desmotubule (DT), runs through the pore. Molecules such as non-cell-autonomous proteins (NCAPs) and small RNAs (smRNAs) can move from cell to cell via the cytoplasmic sleeve (CS) or along the ER membrane. Small molecules can also move via the DT lumen. Callose turnover at the neck region of PD regulates the channel aperture. Various PD-localized proteins have been identified, including Plasmodesmata, myosin, GPI-anchor proteins, callose-binding proteins (PDCBs), and PD-localized proteins (PDLPs). Remorins and GPI-anchor proteins may be associated with sphingolipid- containing microdomains of PM; https://www.researchgate.net

Model illustrating two mechanisms involved in the MP-mediated delivery of viral infectious nucleic acids to PD. (a) In TMV, a VRC produces vRNA, which becomes bound by the 30 kDa MP to form a vRNP complex. The microtubule network might contribute to VRC assembly through the formation of a microtubule-proximal particle. The vRNP complex and/or VRC then moves along the actin/ER network to the PD orifice, where the MP interacts with the SEL binding motif on a PD protein to induce microchannel dilation. The MP – vRNA complex then moves along the surface of the appressed ER to enter the neighboring cell. EB1a and MPB2C act as negative regulators of vRNP complex/VRC delivery to the PD. (b) In PVX, three viral proteins are involved in the delivery of vRNA to PD. Two integral membrane proteins, TGBp2 and TGBp3, form a vesicle – protein complex that can connect to myosin motors. The TGBp1 – vRNA cargo binds to the TGBp2 – TGBp3 complex for delivery to the PD orifice, where the TGBp1 interacts with the SEL binding motif on a protein to induce PD dilation. The TGBp1 – vRNA complex then moves through an opened PD microchannel; the molecular mechanism for this movement remains to be resolved. Finally, TGBp2 and TGBp3 are recycled through the endocytic pathway. Redrawn after Lucas ; https://www.researchgate.net

Regulation of Cell-To-Cell Transport of RNA and Proteins via Plasmodesmata http://www.univie.ac.at/ibmz
In plants homeotic proteins involved in meristem initiation and/or maintenance such as KNOTTED1 (KN1) or which confer and maintain floral meristem identity such as LEAFY are thought to move like viral movement proteins to adjacent cells via intercellular pores formed by plasmodesmata. A number of studies suggest that specific and regulated cell-to-cell transport of proteins and RNA molecules depends on cellular Plasmodesmal pathway receptors. Homeotic transcription factors function within the nucleus, thus, a decision between non-cell-autonomy versus nuclear import has to be made within the cytoplasm. Next, actively transported transcription factors have to interact with specific receptors regulating access to the intercellular transport machinery established by plasmodesmata, which in turn transfers the non-cell-autonomous proteins to neighboring cells via the plasmodesmatal pores. However, limited knowledge is available regarding interaction partners of non-cell-autonomous transcription factors such as KN1-binding proteins and their functional role(s) in cellular distribution.
Recent results indicate that entry into the plasmodesmal transport pathway can be negatively regulated by a novel microtubules-associated protein named MPB2C. Structurally and functionally distinct proteins such as the tobacco mosaic virus movement protein (TMV-MP), KNOTTED1 (KN1) as well as the A. thaliana KN1 homologue SHOOT MERISTEMLESS (STM) interact with MPB2C. Ample presence of the MPB2C prevents cell-to-cell movement of the homeobox transcription factor KN1 and TMV-MP. In addition, we isolated a novel KN1/STM binding protein, KNB36, which is also transported from cell to cell and binds to MPB2C. To exclude that KN1 cell-to-cell transport is simply impaired due to the overexpression of an interacting protein we tested the effect of KNB36 on KN1 transport. In microinjection experiments KNB36 has no effect KN1 cell-to-cell transport. These observations suggest that MPB2C binds a range of structurally distinct proteins and specifically decides/regulates their intracellular distribution and, consequently, also access to the non-cell-autonomous transport pathway established by plasmodesmata. http://www.univie.ac.at/

Potential mechanism for regulating the transport of non-cell-autonomous RNA into the phloem translocation stream. RNA as a long-distance information macromolecule in plants; In this model, non-cell-autonomous RNA molecules contain cis-acting sequence elements, termed 'zip codes', which function in combination with companion cell-specific zip code-binding proteins to direct these transcripts to the companion cell–sieve element plasmodesmata. On entry into the sieve element, the exposed zip code on the non-cell-autonomous RNA molecule is recognized and bound by a competent supracellular transport protein (STP) that then imparts a tissue-specific address to this ribonucleoprotein complex. Competency is imparted to the STP by phosphorylation during, or following, its transport through the companion cell-sieve element plasmodesmata. http://www.nature.com
Phloem is also involved in transporting hundreds of proteins some of them as large as 100kDa and many long non-coding RNAs. One important protein that is transported from leaves to stem apex is FT- Florigin, most coveted flowering component, which eluded plant biologist for many decades. Florigin actually a protein called FT is synthesis in response to flower inducing factors are transported along sieve tube to the base of Apical SAM. Sieve tubes transport not only proteins but also several types of non-coding RNAs such as siRNAs and miRNAs, including mRNAs. Xylem elements are involved in transport from roots to shoot and phloem is involved in transporting from leave to shoots and roots. Transport is uses Ca2+ ions as signaling molecules. Sieve tubes form a network and they are not dead cells, but living for they contain protoplasmic thin layer containing ER and few cellular components at the inner surface of plasma membrane
Sieve plates are porous contain living protoplasmic strands pass through each of them. The sieve plate is also reinforced with callus. Sieve tube cells contain supportive fibers and sclereids. Sieve cells contain central vacuole with longitudinal protoplasmic stands which facilitate transport. Plants utilize the vascular system as long-distance signaling pathways.
Carbohydrate (food) is prepared in mesophyll cells and translocated in the form of sucrose first into leaf veins. The movement of water and dissolved minerals in xylem is always upward (from soil to leaves and stem apexes). The movement of food can be upward plant branched region as well as downwards to roots depending upon the needs and location of the plants.
Components of castor bean plant sieve tube/ companion cell sap are: - Sugars-non-reducing 80-106mg/ml, amino acids 5mg/ml, Potassium 2-4mg/ml, organic acids 2-3mg/ml, Proteins 1-2mg/ml, chloride 0.3-0.6mg/ml phosphate 0.3-0.6mg/ml and Magnesium 0.1mg/ml.

Potential signaling molecules of Xylem (blue) and phloem (red) are shown. Insets show xylem loading and phloem unloading in sink tissues. Signals relay from xylem to phloem, this happens in leaf veinlets. Signals converge by running through stem region along xylem and phloem pathways. http://www.frontiersin.org/

Figure: In the polymer trap mechanism, sucrose diffuses from the bundle sheath cell into the intermediary cell and is enzymatically transformed into oligomers, which are too large to diffuse back. The figure shows the relevant cells inside a leaf with the assumed sugar and water flows. The flows are driven by differences in concentration and pressure between the cells, and influenced by the properties of the interfaces (pore size and density). http://www.fysik.dtu.dk/

SE-CC Complexes in Minor Veins. http://www.waterjournal.org/
(A) Apoplastic loading configuration in Zinnia elegans. The CC possesses numerous wall ingrowths (filled circles) to facilitate apoplastic solute transfer. Bar = 1 μm.
(B) Symplastic loading configuration in Cucurbita pepo. The intermediary cell (IC) is connected to the bundle sheath cells by numerous plasmodesmata (arrows). V, vacuole. Same scale as in (A). (Micrographs courtesy of S. Dimitrovska and A.J.E. van Bel). http://www.plantcell.org/content
Evidence for symport of sucrose and protons has accumulated recently. “These features are satisfied by proton symporters that load se/cc complexes from the leaf apoplasm with sucrose (Riesmeier et al. 1993; Stadler et al. 1995; Truernit & Sauer 1995; Stadler & Sauer 1996; Kühn et al. 1997; Barker et al. 2000; Weise et al. 2000) or with mannitol (Noiraud et al. 2001).” (ibid p41 and references therein) http://www.waterjournal.org/ More recently, Zhou et al. (as reported in Geiger 2011) have proposed a six-state reaction cycle of the proton-coupled disaccharide carrier ZmSUT1 for sucrose loading across membranes in which the sucrose molecule is always transported with a proton through a sucrose/proton symporter. (Geiger 2011, Fig. 4, after Zhou et al. 1997) Geiger concludes that ZmSUT1 is working on the basis of a 1:1 H+ sucrose stoichiometry.
“Amino acid loading into the phloem is also carried out by H+-coupled transport; http://www.waterjournal.org/

A Transfer Cell Three walls of this transfer cell in a pea leaf have knobby extensions that face the cells from which the transfer cell imports solutes. A transfer cell exports the solutes to the neighboring sieve tube element.
Some of these parenchyma cells, called “transfer cells”, are structurally modified for transporting mineral ions from their cytoplasm (part of the symplast) into their cell walls (part of the apoplast). The cell wall that receives the transported ions has many knobby extensions projecting into the transfer cell, increasing the surface area of the cell's plasma membrane, the number of transport proteins, and thus the rate of transport (Figure 36.6). Transfer cells also have many mitochondria that produce the ATP needed to power the active transport of mineral ions. http://www.78stepshealth.us/

The structure of a phloem sieve tube and its companion cells; Note the peripheral layer of cytoplasm against the walls of the sieve tube members (sieve tube elements, sieve elements or sieve tube cells) bounded by a degraded tonoplast (the membrane around the original cell vacuole) which loosely separates the cytoplasm from the lumen. During differentiation, condensed and confined to the margins of the tube and may condensed into P-protein bodies. condensed into P-protein bodies. Smooth endoplasmic reticulum (SER)
Gradually degenerates and may be absent altogether in mature sieve elements.

Phloem is comprised of cells called sieve-tube elements. Phloem sap travels through perforations called sieve tube plates. Neighboring companion cells carry out metabolic functions for the sieve-tube elements and provide them with energy. Lateral sieve areas connect the sieve-tube elements to the companion cells.
Source: Boundless. “Transportation of Photosynthates in the Phloem.” Boundless Biology. Boundless Retrieved on 15 Apr. 2016; httpps://www.boundless.com
Phloem transport is bidirectional. Plant cells use energy stored in the proton gradient and membrane potential to drive the transport of many different solutes. For example, the membrane potential generated by proton pumps contributes to the uptake of K+ by root cells. In the mechanism called anti-port, a transport protein couples the downhill passage of one solute (H+) to the uphill passage of another (NO3−). The "coat-tail" effect of co-transport is also responsible for the uptake of the sugar sucrose by plant cells. A membrane protein co-transports sucrose with the H+ that is moving down its gradient through the protein. Buildup of sugars in sieve tubes builds pressure at the source thus it moves into sites where the pressure is less, thus the flow is from source to sink. http://wonderwhizkids.com/
Determining the structures involved in Translocation ;
Ringing Experiment: To find out whether, the xylem or phloem tissue is involved in translocation, it is possible to remove the cortex and phloem of the stem in the form of a girdle. If the xylem is involved in the transportation, the roots found below the ring should not suffer because the xylem is intact in this experiment. But in this experiment, roots suffer temporarily because; they don’t get adequate supply of food material, for phloem and cortex is removed. So, the food material accumulates at the edge of the ring and with time this region swells into a ridge. This experiment suggests that phloem elements are involved in the translocation of food materials.
Radioactive tracer experiment
If radioactive traces like 14CO2 or (14C) sucrose is fed to leaves and chased, i.e. followed with time, it is possible to follow the pathway through which the photosynthate or sucrose is moving.

Organic solute transport to sink such as tubers in plants; www.uic.edu



Cross section of P-sieve tube element; enriched cytoplasmic elements at the periphery; http://botit.botany.wisc.edu/Anatomy

Radioactive tracers used to locate solutes transfer; www.saylor.org
Tracer studies with autoradiography or sap analysis clearly shows that the sieve tubes are the structures through which organic materials are translocated. This method can be used to determine even the rate of translocation.
Experiments with aphids provide the information of the contents sieve tube translocates.
Structure and organization of sieve elements:
Among the different phloem elements sieve tube cell and companion cell form an inseparable pair of cells. Both sieve tube cell and companions are derived from the same mother cell. And sieve tube acts as the main structure through which the organic solutes are translocated.
Anatomical structures involved in Translocation of solutes:

Sieve tubes associated with companion cells; www.cell.com

www.driverlayer.com
Though both cell types are derived from the same procambial cells, sieve cell undergoes considerable expansion in length and breadth. In order to keep pace with the growth of the sieve cell, the companion cells divide once, or twice for every single sieve tube that develops. Due to expansion of the sieve cells, a large central vacuole develops with thin peripheral protoplasm. Cross walls between sieve cells gets perforated considerably around plasmodesmata. Nucleus, Ribosomes, Tonoplast are degraded to provide low resistance. Mature sieve tube cells contain peripheral protoplasm, mitochondria, ER, sieve element plastids and most importantly “P-proteins (Now phloem specific proteins are considered as the artifacts developed during cell preparation). But companion cells are strongly connected to sieve tube cells symplastically, and it is very active. Companion cells are rich protoplasm with many mitochondria and ribosomes and it provide required materials for the functioning of sieve tubes. Sieve tubes contain distinctive network of protein filaments.

Companion cells derived from the same mother cell is associated with sieve tube cells symplastically with specialized “Pore Plasmodesmata” provides a continuum; Note; each sieve tube cell is in contact with at least four companion cells around each sieve tube elements; http://virtualplant.ru.ac.za/

https://online.science.psu.edu; www.gopixpic.com

Schematic drawing of sieve tubes in Vicia faba (broadbean). C, callose; CC, companion cell; CP, forisome; CW, cell wall; ER, endoplasmic reticulum; M, mitochondria; N, nucleus; P and Pl, plastids; PP, parietal protein; PPU, pore plasmodesma unit; SE, sieve element; SP, sieve plate; V, vacuole. From Knoblauch and Van Bel (1998) Sieve tuibes in action. Plant Cell 10, 35-50. Copyright American Society of Plant Biologists; http://public.wsu.edu/

Schematic drawing of sieve tube structure in Arabidopsis thaliana: C = chloroplast, Cl = clamp proteins, ER = endoplasmic reticulum, EV = electron dense vesicles, GM = ground matrix, M = mitochondrium, N = nucleus, P = plastid, SR = SEOR1 filaments, V = vacuole. From- Froelich et al. 2011. Phloem ultrastructure and pressure flow: SEOR protein agglomerations do not affect translocation. Plant Cell. Plant Cell doi/10.1105/tpc.111.093179 Copyright American Society of Plant Biologists; http://public.wsu.edu/

Callose formation in bamboo. A) Sieve plate in the phloem of a vascular bundle of bamboo. B) Sieve plate in bamboo before injury. C-F) Callose formation around pores after 3 min (C), 10 min (D,E) and 20 min (F). From Mullendore et al. (2010) Sieve tube geometry in relation to phloem flow. Plant Cell 22, 579-593. Copyright American Society of Plant Biologists; http://public.wsu.edu/

http://www1.biologie.uni-hamburg.de/

Plasmodesmata interconnecting between sieve elements and companion cells; you find characteristic branching of plasmodesmata within companion cell; http://plantsinaction.science.uq.edu.au/

RNA as a long-distance information macromolecule in plants; William J. Lucas, Byung-Chun Yoo and Friedrich Kragler; http://www.nature.com/
Non-cell-autonomous RNA molecules contain cis-acting sequence elements, termed zip-code which function in combination with companion cells–specific binding proteins to direct these transcripts to companion cell-sieve element plasmodesmata. On entry into sieve elements the exposed zip code on the non–cell-autonomous RNA molecule is recognized and bound by competent supracellular transport protein (STP) that then imparts a tissue-specific address to this ribonuclear protein complex. Competency is imparted to the STP by phosphorylation during, or the following, its transport through the companion cell-sieve element plasmodesmata.

Schematic Representation of the Histology of the Sieve Element/Companion Cell-Complex in Vicia faba.
Four sieve elements (SE) connected by sieve plates (SP) are shown with their adjacent companion cells (CC). Only the companion cells contain nuclei (N), vacuoles (V), and chloroplasts (C); mitochondria (M) and endoplasmic reticulum (ER) are also indicated. Typical features of the sieve elements in this species are the sieve element plastids (Pl), the parietal P-proteins (PP), and the P-protein crystalloids (PC). One crystalloid is shown in the dense state, in which it permits the sieve element contents to flow past it. The other is depicted in the dispersed state (DPC), in which it blocks the tube. The direction of flow is downwards, as indicated; http://www.1biologie.uni.hamburg.de

Phloem loading: To establish a high-pressure potential in sieve tube members near
source cells, large amounts of sugar have to be transported into the phloem sap-enough
to raise the solute concentration of sieve tube members. -Phloem loading is
thought to depend on a proton pump, (H+-ATPase) and a cotransporter, a protein
that serves as a conduit for protons and sucrose to enter the cell together.
-Uses energy (ATP);
Phloem
unloading: Occurs when cells take up
sucrose.
-It depends on the sink of where the sucrose is
leading too.
-Energy use varies between tissues:
energy required in storage tissues but not in growing tissues.

SE-CC Complexes in Minor Veins. (A) Apoplastic loading configuration in Zinnia elegans. The CC possesses numerous wall ingrowths (filled circles) to facilitate apoplastic solute transfer. Bar = 1 μm. (B) Symplastic loading configuration in Cucurbita pepo. The intermediary cell (IC) is connected to the bundle sheath cells by numerous plasmodesmata (arrows). V, vacuole. Same scale as in (A). (Micrographs courtesy of S. Dimitrov ska and A.J.E. van Bel). http://www.plantcell.org/content;http://plamt.info; . http://www.78stepshealth.us/
Some of these parenchyma cells, called transfer cells, are structurally modified for transporting mineral ions from their cytoplasm (part of the symplast) into their cell walls (part of the apoplast). The cell wall that receives the transported ions has many knobby extensions projecting into the transfer cell, increasing the surface area of the cell's plasma membrane, the number of transport proteins, and thus the rate of transport (Figure 36.6). Transfer cells also have many mitochondria that produce the ATP needed to power the active transport of mineral ions.

Transfer Cell Three walls of this transfer cell in a pea leaf have knobby extensions that face the cells from which the transfer cell imports solutes. A transfer cell exports the solutes to the neighboring sieve tube element. Some of these parenchyma cells, called transfer cells, are structurally modified for transporting mineral ions from their cytoplasm (part of the symplast) into their cell walls (part of the apoplast). The cell wall that receives the transported ions has many knobby extensions projecting into the transfer cell, increasing the surface area of the cell's plasma membrane, the number of transport proteins, and thus the rate of transport (Figure 36.6). Transfer cells also have many mitochondria that produce the ATP needed to power the active transport of mineral ions. http://www.78stepshealth.us/
Transfer cells- are specialized parenchyma cells that have an increased surface area, due to infoldings of the plasma membrane. They facilitate the transport of sugars from a sugar source, mainly mature leaves, to a sugar sink, often developing leaves or fruits. They are found in nectaries of flowers and some carnivorous plants. Transfer cells are specially found in plants in the region of absorption or secretion of nutrients; Wall ingrowth formation in transfer cells: novel examples of localized wall deposition in plant cells. The formation of wall ingrowths increases plasma membrane surface areas of transfer cells involved in membrane transport of nutrients in plants. Construction of these ingrowths provides intriguing and diverse examples of localized wall deposition. Flange wall ingrowths resemble secondary wall thickenings of tracheary elements in morphology and probable mechanisms of deposition. By contrast, reticulate wall ingrowths, deposited as discrete papillate projections, branch and fuse to create a fenestrated wall labyrinth representing a novel form of localized wall deposition. Papillate wall ingrowths are initiated as patches of disorganized cellulosic material and are compositionally similar to primary walls, except for a surrounding layer of callose and enhanced levels of arabino-galactan proteins at the ingrowth/membrane interface. How this unusual form of localized wall deposition is constructed is unknown but may involve constraining cellulose-synthesizing rosette complexes at their growing tips. http://www.ncbi.nlm.nih.gov/ https://en.wikipedia.org
Transverse and vertical sections of Phaseolus vulgaris stem; these are C14 labelled sections; http://plantsinaction.science.uq.edu.au/

Transcerse section showing apart of vasculature of Cucurbita stem, the right ones are TEM pictures of sieve tubes associated companion cells. Seive plates have membrane lined spaces for the transport of solutes aided by plasmodesmata. Margins of the cells are lined with thin cytoplasm with low density organelles. Associated c ells like companion cells are rich in cytoplasm; http.htpp:// Plants in action .science.ug.edu.au.
Left- TEM and the right one is diagrammatic interpretation of secondary plasmodesmata interconnecting a mature sieve tub and its companion cells; http://plantsinaction.science.uq.edu.au/
Transfer cells are ubiquitous plant cells that play an important role in plant development as well as in responses to biotic and abiotic stresses. They are highly specialized and differentiated cells playing a central role in the acquisition, distribution and exchange of nutrients. Their unique structural traits are characterized by augmented ingrowths of invaginated secondary wall material, unsheathed by an amplified area of plasma membrane enriched in a suite of solute transporters.

Figure 1.16 Plasmodesmata connect the cytoplasms of neighbouring cells facilitating cell-to-cell communication and solute transport (source: Taiz L., Zeiger E., 2010) Solutes move through both apoplast and symplast plasmodesmata, cylindrical pores 20 to 60 nm in diameter

http://image.slidesharecdn.com/


http://image.slidesharecdn.com/

http://image.slidesharecdn.com/
Translocation of organic solutes from source to the regions needed such as leaves to stem tip and root tip. Pressure flow hypothesis was first proposed by Ernst Munch in 1926’. According to the hypothesis, more or less accepted by the present authors, dissolved sugars are loaded into sieve tubes by a combination of active transport and mass flow generated by active transport. Leaves synthesize glucose which is converted to sucrose (the stable compound for transport). Sucrose is actively loaded into sieve tube via modified companion cells called ‘Transfer Cells’. The said modified transfer cells (modified companion cells) with numerous ingrowths (increase surface area) are rich in mitochondria which provide ATPs for active loading by what is termed as cotransport. Sugar loading is by chemiosmatic mechanism. Proton pumps hydrogen ions out of cell membrane resulting in a proton gradient across the membrane. The cotransport protein then transports Hydrogen ions down its gradient back to the cell and sugar is transported into the cell. ATP provides energy to pump protons out of sieve cells, producing proton gradient that drives uptake of sugar. Thus, sugar accumulates in large amounts, 2-3 times the surrounding cells. This leads to water movement into sieve cells from xylem elements and causes increased turgor pressure (hydrostatic pressure) inside the sieve cells at the base (remember sieve cells are organized one above the other in long tubular fashion). At the destination the sugar is unloaded. Mass flow shows the fluid movement from high hydrostatic pressure to the region of low hydrostatic pressure.
Materials translocated in the phloem;
Phloem sap can be collected from aphid stylets or, alternatively, from some plants by simply making an incision into the bark. If done carefully, to avoid cutting into the underlying xylem, the incision opens the sieve tubes and a relatively pure exudate can be collected in very small microcapillary tubes for subsequent analysis. As might be expected, the chemical composition of phloem exudate is highly variable. It depends on the species, age, and physiological condition of the tissue sampled. Even for a particular sample under uniform conditions, there may be wide variations in the concentrations of particular components between subsequent samples. For example, an analysis of phloem exudate from stems of actively growing castor bean (Ricinus communis) shows that the exudate contains sugars, protein, amino acids, the organic acid malate, and a variety of inorganic anions and cations. The inorganic anions include phosphate, sulphate, and chloride – nitrate is conspicuously absent – while the predominant cation is potassium. Some plant hormones (auxin, cytokinin, and gibberellin) were also detected, but at very low concentrations. The principal constituent of phloem exudate in most species is sugar. In castor bean it is sucrose, which comprises approximately 80 percent of the dry matter. A survey of over 500 species representing approximately 100 dicotyledonous families confirms that sucrose is almost universal as the dominant sugar in the phloem stream.
It is interesting to speculate on why sucrose is the preferred vehicle for long-distance translocation of photo-assimilate. One possibility is that sucrose, a disaccharide, and its related oligosaccharides are nonreducing sugars. On the other hand, all monosaccharides, including glucose and fructose, are reducing sugars. Reducing sugars have a free aldehyde or ketone group that is capable of reducing mild oxidizing agents. Some oligosaccharides, such as sucrose, are nonreducing sugars because the acetal link between the subunits is stable and nonreactive in alkaline solution. The exclusive use of nonreducing sugars in the translocation of photo-assimilate may be related to this greater chemical stability.
The movement of sugars such long distance is a cellular phenomenon is not yet clear. It is believed that it is due to electro-osmosis, use K ions; on the contrary another hypothesis is cytoplasmic streaming, where the fluid moves across sieve plates, for which energy is provided by ATP from phloem cells.
More recently trans-cellular strands that contain contractile proteins are found in sieve elements. They are found as fine cytoplasmic strands where the solute is moved one tube to the next by –constriction+ relaxation/peristaltic movement in a a wav pattern, by what is termed as rhythmic contraction and relaxation causes facilitated movement.
Sucrose transporter is seven-transmembrane protein (42kDa) in sugar beet, 55kDa in spinach, 54.5kDa in Arabidopsis.

www.cnx.org; http://www.uic.edu

Transcellular strands in sieve tubes involved in solute transportation; Transcellular strands are nothing but a bundle of P-protein filaments ~30nm thick, not all of them; http://www.jstor.org/1976

http://igbiologyy.blogspot.com
It is during the enlargement; the nuclear membrane disappears and the chromatin material breaks up and gets distributed through out the protoplasm. Almost at this juncture a massive number of mRNAs that are transcribed by the chromatin bits left free, perhaps stored to used when required. [In the case of RBC mRNA remain active for more than 120 days and human erythrocytes lack nuclei similar to sieve cells. The proteins that are synthesized due to such transcription and translational activity are none other than tubulins, which soon organize into longitudinally oriented microtubules. The microtubules are also associated with microfilaments and smaller microtrabcular structures. Such Protein complex is called phloem proteins. The longitudinally oriented parallel bundles of microtubules and microtrabacular filaments actually traverse across the transverse septa called sieve plate which is highly perforated.
Movement of water shows both apoplastic and symplastic type of movement. Apoplastic- movement of water and solutes through spaces found in cell wall and intercellular spaces... Transport is rapid for less resistance. Symplastic movement through plasmodesmata that form living membranous tubes found in between sieve tube across sieve plates.

Sucrose and monosaccharide transporters mediate long distance transport of sugar from source to sink organs and constitute key components for carbon partitioning at the whole plant level and in interactions with fungi. Even if numerous families of plant sugar transporters are defined; efflux capacities, subcellular localization and association to membrane rafts have only been recently reported. On the fungal side, the investigation of sugar transport mechanisms in mutualistic and pathogenic interactions is now emerging. Here, we review the essential role of sugar transporters for distribution of carbohydrates inside plant cells, as well as for plant–fungal interaction functioning. Altogether these data highlight the need for a better comprehension of the mechanisms underlying sugar exchanges between fungi and their host plants. Sugar Transport in plants- Joan Dokly et al. Trends in Plant science; http://www.cell.com/

Sugars from mesophyll cells are translocated into phloem companion cells. This creates reduced water potential, thus water from xylem enters into companion cells/sieve tubes thus creates pressure potential, which pushes the organic solutes towards the lower potential such as root, tubers and stem apex; The question bidirectional movement of organic solute in the same sieve tube is questionable. If this has be explained, one has to visualized as having transport tracts where the food is carried as found in other systems, where microtubules are involved in cargo transport, but no such organized microtubules or actin fibers are found in sieve tubes, or P-proteins involved in the transport is not known.www.plantphys.info

Remi Lemoine and fourteen others http://journal.frontiersin.org/
Comparison of source-to-sink sugar transport in symplastic and apoplastic active phloem loaders: Sucrose available for export from mesophyll cells (MC) results from a balance between storage in the vacuoles and sequestration as starch in the chloroplasts. Sucrose can reach the sieve tubes through plasmodesmata that allow for its diffusion from cell to cell in species like cucurbits. Sucrose is converted to larger molecules (RFOs) by the sequential addition of galactosyl residues in modified companion cells (CC) called intermediary cells. The larger molecules cannot move back to phloem parenchyma cells (PP) and are transferred and accumulated in sieve tubes. In apoplast-loading species, sucrose reaches phloem parenchyma cells through plasmodesmata. Sucrose is loaded and accumulates in the phloem by passing through the apoplast between the PP and the CC. The major players are presented in the enlargement of that area. Sucrose enters the apoplast through facilitators of the SWEET family (pale green circle) and is accumulated in the companion cell by a proton/sucrose co-transporter of the SUT1/SUC2 type (green circle). The energy necessary for the co-transport is provided by an H+/pumping ATPase (black circle) which establishes a proton gradient and a trans-membrane potential regulated by potassium channels of the AKT2/3 type (white circle). In Solanaceous species, SUT1 transporters are localized at the plasma membrane of sieve elements (not shown). Polyols can also be transported into the phloem, with specific transporters located in the plasma membrane of CC (not shown). A high hydrostatic pressure is generated in the sieve tubes of the collection phloem and water from the xylem is attracted. Sucrose, RFOs and polyols are transported in the sieve tubes to the sink organs in the transport phloem. All along the path, they can be leaked from and reloaded into the phloem via the same mechanism (not shown). Sucrose is unloaded into the release phloem where the hydrostatic pressure is supposed to be lower. Sucrose can be unloaded through a symplastic pathway or through an apoplastic pathway. In the latter case, sucrose is unloaded into the apoplast through specific carriers which can be of the SUT1/SUC2 type (green circle; Carpaneto et al., 2005). Sucrose is then taken up by sink-specific sucrose carriers of the same SUT1/SUC2 (light green circle) or converted to hexoses by a cell-wall invertase (CWInv). Hexoses are then taken up by specific carriers at the plasma membrane (orange circle) or at the tonoplast level (yellow and brown circles). Sucrose in sink cells can be metabolized (growing sinks) or stored as starch in amyloplasts, or imported into the vacuoles (red circles) and further converted to hexoses by a vacuolar invertase (VInv) by Remi Lemoine and fourteen others http://journal.frontiersin.org/.

Model of the plant’s responses to mineral nutrient deficiency. (A) Response to nitrate and phosphorus deficiency: deficiency in nitrogen and phosphorus leads to reduced photosynthesis, accumulation of sugars in source leaves, increased carbon allocation to the roots and a higher root/shoot ratio. Moreover, phosphorus limitation induces an adaptation of the root system architecture: root hairs initiate and elongate, which increases the root surface area. AtSUC2 (green circle) is a component of the sugar-signaling pathway in the response to phosphorus starvation. (B) Response to magnesium and potassium deficiency: Mg deficiency increases the concentration of soluble sugars and starch in leaves and reduces leaf growth. Mg deficiency impacts sugar metabolism, as well as sucrose export to the roots. Mg deficiency reduces the Mg-ATP availability and the activity of H+-ATPase, thus reducing the driving force for sucrose phloem loading. AKT2/3 potassium channels affect sugar loading and long-distance transport by regulating the H+/sucrose transporter. Conversely, K+-limitation rarely results in starch accumulation. MC, mesophyll cell; CC, companion cell; PP, parenchyma phloem; MC, mesophyll cell. http://journal.frontiersin.org/
Thus the proteins provide a kind of continuum between the longitudinally aligned sieve tube cells. Once the P. protein complex develops the distinction between the peripheral cytoplasm and the central vacuole disappears. Interestingly, the mRNAs produced in sieve tubes have longer life period of 2-5 months. Besides the above said components, sieve cells also possess slime bodies, mitochondria and proplastids and traces of endoplasmic reticulum.
The transverse septa found between two adjacent sieve cells are highly perforated and callose is deposited on either side of the septa in the form of cushions. Through each of these pores, more than 100-150 parallel array of P. proteins pass through, thus the cellular components pass through from one cell to the other. Actually, recent investigations suggest that the transcellular strands that traverse the sieve plates are made up of plasmodesmata, an extension of ERs loaded with actin and its associated motor proteins such as Myosins.
Companion cells, in spite of deriving from the same precursor cell, does not undergo such transformation as in the case of sieve cells, but it undergoes one or two transverse mitotic divisions and the cells retain all its cytoplasm without any vacuolar formation. More than that companion cells contain a large number of active mitochondria. The lateral wall between the companion cell and sieve cell is perforated through which a large number of plasmodesmata pass through, so that substances can pass through from one cell to another. Thus, companion cells provide the necessary energy for the efficient function of the sieve tube.
Sieve tubes are arranged longitudinally one above the other so as to form a continuous column, which runs from one end of the plant to the other end. At the nodal regions, among the sieve tubes which emerge from the petiole, some run upwards in the stem and some downwards i.e., towards the root. Thus, sieve tubes provide a specialized network of longitudinally oriented living micro pipelines for the transportation of food materials from the leaves to other regions of the plant body. Notably, the presence of phloem parenchymatous cells around sieve tube it is very interesting, because they are in contact with both sieve tubes on one side and mesophyll cells at the other end of the leaves. Structurally and functionally, they look like transfer cells. Further more, water containing xylem elements are also in close proximity with the sieve cells.
Sieve tubes are now known to conduct signal transductions generated by leaves in response to Photoperiodism. Some of the signal molecules produced in leaves, especially mesophyll cells are translocated to sieve tubes, it is through them the signal molecules are transported to shoot meristems, where the vegetative meristems get transformed into floral meristem. It is now known that light induced “florigin” an elusive flowering substance is none other than a protein called FT (floral locus T). Sieve tubes are the channels through which these molecules are transported; now it is an established fact.

High-pressure manifold model that describes phloem transport from source to sink and partitioning of resources (water and dissolved solutes) between sinks. Sucrose is loaded (brown arrows) into the collection phloem (minor veins; yellow borders) of source leaves to high concentrations (dark purple) that drives an osmotic uptake of water (blue arrows). Walls of the collection phloem SEs resist the volume change with a consequent development of high hydrostatic pressures (example given, 1.4 MPa and see Table 1). STs form conduits interconnecting sources (dark green) to sinks (light purple) in a super cellular symplasm comprising collection, transport (dark green) and release (khaki) phloem. Hydrostatic pressures, generated in collection phloem SEs, are rapidly transmitted throughout the entire ST system and maintained by pressure-dependent retrieval of leaked sucrose and hence water (curved brown and blue arrows respectively). Thus, STs are conceived to function as high-pressure conduits rendering resources equally available throughout a plant. Transported resources are unloaded from the release phloem SE/CC complexes as a bulk flow through high resistance (low conductance Lo and see Equation 2) plasmodesmal pathways into the sink cells. SE/CC unloading imposes the greatest constraint over resource flow through the source-transport-sink pathway. As a consequence, resource partitioning between sinks is finely regulated by their relative hydraulic conductance of plasmodesmata linking SE/CC complexes with the surrounding phloem parenchyma cells. http://www.frontiersin.org/

Turgor homeostasis model of phloem unloading in developing seeds of grain legumes. Sucrose uptake by, and storage, in cotyledons is coupled through a negative feedback transcriptional regulation of sucrose transporter activity mediated through intracellular sucrose levels. Activities of seed coat sucrose effluxers are coordinated with cotyledon demand by a turgor-homeostat mechanism that osmotically (π) detects alterations (error signal) in apoplasmic sucrose pool sizes as a deviation from a turgor (P) set point. Symplasmic unloading from release phloem SE/CC complexes by bulk flow is regulated by plasmodesmal hydraulic conductance’s (Lo) under control of the turgor homeostat functioning as a central control hub to regulate resource flow from SE/CC complexes to ultimate storage in cotyledons.
Rate and Magnitude of translocation:
The rate of translocation of organic food materials can be determined by providing a pulse of 14C sucrose or 32 P-ortho phosphates to leaves and then chases it. Results indicate that the rate of translocation ranges from 55 cm to 290 cm/hr. which again depends upon the season, the time of the day, temperature and other factors. In spite of the presence of a large number of sieve plates across, there are 1000-2000 sieve plates for every 1 cm length of the stem; the rate of translocation is 500-1000 times faster than the rate of simple diffusion. However, not all components show the same rate of translocation. For example, carbon compounds like sucrose, glucose and fructose move faster than phosphates and tritiated water. Different amino acids exhibit different rates of movement within the same plant body.
Use of radioactive isotopes has come very handy in obtaining accurate qualitative and quantitative data. This can be calculated based on the amount of radioactive material fed at the supply end i.e. Leaves and the amount of radioactive material received at the receiving ends like stem apex, root apex, fruits and tubers. This way it has been estimated that the amount of organic carbon source translocated ranges from 200 mg to 800 mg per hour. Particularly plants like Cocos nucifera, Borasus, Phoenix and such palm plants transport enormous amount of sap ranging from 20 ml to 200 ml per hour. More than 18% of the sap in them is sugar.
CHEMICAL COMPOSITION OF TRANSLOCATE
Aphid stylets are the best to obtain the cell sap from the sieve tubes. Once the insects are allowed to bite into the stem, they pierce their stylets into the stem so as the ends of sty lets reach the sieve tubes exactly. Then the insects can be etherized and heads can be severed, so as to allow the sap to exudates out of the free ends of the stylets. Chemical analysis of such sieve tube exudates reveals the presence of various organic compounds like sucrose, glucose, fructose, almost all amino acids, enzymes, vitamins, phytohormones, sugar alcohols, oligo saccharides like stachyose, raffinose and RNA etc.
Recent work shows leaves produce FT transcripts, under the influence light period, which are transported to leaf companion cell. From there the FT mRNA with its bound proteins are transported to apical meristems where they act to produce the required components of inducing flower. But more than 80% of the total translocates is sucrose. However, the glucose and fructose found in the sap are rather hydrolytic products of sucrose than the actual translocates from the source.
Phloem exudates have been analyzed on 2-D acrylamide gel and the same is shown below.

Two-dimensional polyacrylamide gel electrophoresis separation of proteins in phloem exudate from Lupinus albus. The gel was developed in the first dimension by isoelectric focusing with a linear pH gradient of 3-10 followed by separation due to differences in molecular mass. The positions of mass standards are shown on the right-hand side of the gel. After staining with Coomassie Blue to locate the spots they were excised for digestion with trypsin. The peptides were then analyzed by partial sequence determination using MS/MS and identified using database searches. (Courtesy of Craig Atkins); http://plantsinaction.science.uq.edu.au/
DIRECTION OF TRANSLOCATION
Organic solutes are translocated from the region of synthesis or storage to the region of need like shoot and root apexes, developing leaves, flowers and fruits. It is interesting to determine the direction of the translocates orginating from a leaf. If radioactive (14C) sucrose is supplied to leaves situated at different positions on the stem, it is possible to follow the direction of the radioactive sucrose precisely. Such experiments reveal that the leaves situated near the shoot apex translocate most of their organic solutes towards shoot apex. Similarly, leaves located at the base of the stem translocate food towards the root. But the leaves found in between them, translocate upwards as well as downwards. However, the bidirectional movement changes with time as more and more leaves are added to the young growing stem and its branches. But the bidirectional movement posses certain problems like, whether the bidirectional flow takes place in different sieve tube assemblies or in the same sieve tube. Some physiologists assumed that as sieve tube is living, it exhibits bidirectional protoplasmic streaming in the same cell, but this view has no supporting evidences. Nonetheless, it is now accepted that the bidirectional movement is due to the transport of solutes in different sieve tube assemblies which are organized into parallel arrays. But recent investigations into microtubule and actin-myosin mediated transport demonstrates that two different microtubular and actin-myosin complex units can transport materials in two different directions. However, one complex shows one directional flow. Interestingly, in germinating seeds or plantlets sprouting from tubers, it is observed that the food material always moves from the source to the terminal regions of the plant body where growing structures are located.
Though most of the food material is translocated vertically through the longitudinally oriented sieve tubes, it is not hard to imagine the lateral or radial transportation of solutes into the cells found in the cortical cells found around the phloem elements. This lateral diffusion is facilitated by the fact that they are in contact with sieve elements. More than that, the lateral diffusion is absolutely essential for their normal metabolic activities and survival.
Factors that control translocation:
Light and Darkness: Light has many effects on biological systems of which photosynthesis is very important. Translocation of food in plants is also influenced by the presence or absence of light. If radioactive 14C sucrose of known quantity is fed to the leaves of two different plants of the same age and then one plant is kept in dark and the other in light either for short duration or longer duration the recovery of radio active isotopes show greatest amounts of radioactivity n the roots, of the plant that is kept in dark, than the plant kept in the light. Though there is a general tendency for the movement of more food material towards roots than shoots, light favors greater movement of organic solutes towards shoot apex and darkness favors the opposite.
The action spectrum of the light that favors translocation has been found to be red and blue which are acts as the action spectrum for photosynthetic reactions. Interestingly, most of the glucose synthesized by leaves during the day is stored as starch and the same is converted into sucrose at nights and transported out of the leaves. This observation can be tested by keeping the plant in dark for 24-48 hours. Afterwards, if leaves are tested for starch with KI, they do not show any coloration. But the leaves of the plants exposed to light from morning to evening show maximum deep blue color for KI test than the leaves exposed to light for one or two hours. The above observations clearly suggest that during night plants translocate most of their food materials from the leaves to other regions, but during day time they do translocate some food materials, but most of it is stored in the form of starch.
Temperature: Translocation is greatly affected by the change in the temperature, which means, the process is a facilitated process and it needs metabolic energy. If the petiole through which organic materials are translocated is cooled by water jackets, translocation is greatly affected. Similarly, if roots or stem tips are subjected to cold temperatures, the translocation of solutes towards them is severely inhibited. On he contrary, if the temperature is increased, the rate of translocation also increases. However very high temperature is fatal, but, certain cold resisting plants like sugar beet are capable of adapting to cold conditions by producing antifreeze proteins and maintain the normal flow of food materials even at freezing temperatures.
Effect of Phytohormones: Growth promoting hormones like indole acetic acid Gebberllins and cytokinins are found to accelerate the rate of translocation. As the above said hormones activate cellular metabolic activities, cell division and cell elongation in the apexes and other growing tissues, the said structures need greater amount of organic solutes energy and structural components. Hence these regions act as sink and organic solutes move in to them with greater facility. Though individual hormones have different but specific effects on plant growth, they accelerate the rate of movement of materials from the source to the sink. For example, IAA increases the rate of movement from the leaves to other regions. Cytokinin favors the translocation of sucrose out of the leaves, but preserves nitrogenous compounds within the leaves. On the other hand, if the same hormone is applied to the roots, it favors as well as accelerates the movement of sucrose, amino acids and other compounds towards roots.
To substantiate the favorable effects of phytohormones on translocation, if an agar block containing IAA or GA is placed over the decapitated stem and 14C sucrose or 14CO2 is fed to the leaves, the sucrose or the photosynthate found in leaves moves towards the region at which hormones is supplied. Phytohormones, have stronger influence not only on the direction of translocation, they also accelerate the rate of translocation.
Effect of minerals: Among all the essential nutrients, boron has been found to facilitate the movement of sucrose. The absence of boron reduces the rate of translocation significantly. Whether boron forms an ionizable complex with sucrose or it facilitates the formation of more of sucrose for transport is not clear. Phosphate has also been implicated in this process but not much is known, how phosphate facilitates or affects the process of translocation. Even K+ ions and other metallic ions have thought to play an important role in electro-osmosis, but their exact mechanism is not substantiated by any reasonable experiments.
Effect of Concentration Gradient:
Translocation between the supply end and the receiving end is always governed by the concentration gradient. Steeper the gradient, greater is the rate of translocation and vice versa. Such a gradient mediated movement of solutes can be demonstrated by cutting off the leaf which acts as the supply end. By removing the leaves, the gradient will be abolished and automatically the transportation comes to stand still. Generally, at the supply end, sieve tubes contents show greater positive pressure and the receiving ends like stem apex, root apex, developing flower or fruit show negative pressure. This provides a motive force for the rapid movement of organic solutes from leaves to the other said regions called sinks.
MECHANISM OF TRANSLOCATION
Various theories have been proposed from time to time, but there is not even a single theory which can explain the phenomenon completely and explicitly. Theories like ‘active diffusion’, protoplasmic streaming proposed by Devries and Curtis, electro osmosis by Spanner and Jones, transcellular streaming by Thiane and Ganny, mass or pressure flow by Munch are based on certain observations which are incomplete in their explanations. Sometimes the assumptions are misguided. Still, most of the above said theories have greatly contributed in understanding the mechanism of translocation, because they have explained one or the other factual observations. But in recent years, the understanding of sieve cell at the ultra structural level, and the use of radioactive tracers, have helped in reconsidering and remodeling the original individual concepts into an unified mechanism, for the entire process is not operated by a single force or a single event, it is a combination of many forces which act as a unified mechanism which operate at different levels or steps; such as vein loading, development of mass pressure and active transportation, etc.
VEIN LOADING
At nights starch found in plastids are first degraded into glucose and 50% of it is converted to fructose 6P. Even during day periods, certain amount of glucose produced by photosynthesis is converted to Fructose 6P. The required glucoses are activated by uridine diphosphate to uridine diphosphate glucoses. Then UDPG combines with fructose phosphate to produce sucrose phosphate.
The enzyme responsible for this process is sucrose synthetase. This process takes place within the plastids.
Starch - Glucose
Glucose -Glucose 6P
Glucose 6P -Fructose 6P
Glucose 6P+U - UDPG+2Pi
UDPG + Fructose 6P-->> Sucrose-P + UDP

Sucrose

Mechanisms of solute transport. Three distinct types of passive transport, as well as active transport, are illustrated. Passive transport is the movement of solute across a membrane down an electrochemical gradient (from the side of the membrane with a high concentration of solute to the side with a low concentration). Active transport is the movement of solutes across a membrane against an electrochemical gradient (from the side of the membrane with a low concentration of solute to the side with a high concentration), which requires energy. Simple diffusion is the unassisted diffusion of a lipid-soluble solute (shown as blue dots) across a lipid bilayer. Transport via an ion channel involves a protein-lined pore (shown in purple) spanning the lipid bilayer through which select ions (shown as green dots) are transported. Facilitated diffusion involves transport of a solute (shown as green triangles) across a membrane by a carrier protein (shown in purple). Active transport involves transport of a solute (shown as green squares) against an electrochemical gradient by a pump protein (shown in purple). This process requires energy. https://wikispaces.psu.edu

The H+/Sucrose pump; www.uic.edu

Four different mechanisms of active transport. Active transport is defined as the transport of solute against an electrochemical gradient, so energy is required. The ATP-dependent pump uses the energy derived from ATP hydrolysis to ADP and inorganic phosphate (Pi) to drive the transport of a solute (green circle) against its electrochemical gradient. The light-driven pump uses the energy of absorbed photons of light to transport a solute (blue circle) against its electrochemical gradient. The symporter transports two different solutes (orange circle and square) in the same direction across a membrane. One of the solutes (e.g. the orange circle) is transported against its electrochemical gradient, hence this is active transport. This transport requires the free energy released from the movement of a second solute (e.g. the orange square) down its electrochemical gradient. The antiporter transports two solutes (purple circle and square) in opposite directions across a membrane. The transport of one solute is against its electrochemical gradient (e.g., the purple circle). This is driven by the coupled transport of a second solute (e.g., the purple square) in the opposite direction, down its electrochemical gradient; https://wikispaces.psu.edu
Sucrose P thus produced is released into cytoplasm. The specific carrier’s pick up the sucrose molecules and transport the same from cell to cell towards vascular bundles. The protoplasmic strands found as inter cellular connecting strands are very active and help in the transportation of materials from one cell to another. Finally, sucrose is transported into vascular bundles, where parenchymatous cells associated with sieve tubes greatly help in loading the sucrose into relatively empty sieve tube cells. Interestingly, in the mesophyll tissues where the veins end or begin the sieve tubes are invariably kept empty so as to draw photosynthates towards the main translocation stream. So the process of loading of veins goes on and on, however vein loading is more active during night than at daytime.

DEVELOPMENT OF MASS PRESSURE
As several hundreds and thousands of sieve tubes, located at the vein ends, are loaded with sucrose, a steep DPD gradient develops between the sucrose laden sieve tube cells and water containing xylem elements. Automatically, water is drawn rapidly into sieve cells enmass.


Munich's "Pressure Flow" model (1927).
Munich's model best describes the movement in the phloem. His model suggests that there is a turgor-pressure gradient that drives the directional mass flow of the solutes and water through sieve tubes of the phloem.

This can be demonstrated with an osmometer permeable only to water filled with a high concentration of solutes in one arm as shown below. When the osmometer is put in distilled water, the water potential is less than that of surrounding water in the one arm, and water will enter by osmosis which the generates a turgor pressure, The solutes are carried by bulk flow when the water moves in. Eventually this process will stop when the pressure throughout equalizes. http://www2.mcdaniel.edu/

Using this analogy, phloem transport can be explained as follows:
1. Sucrose is loaded into sieve tubes by active transport. 2. Water potential decreases and water enters sieve tubes by osmosis, turgor pressure. 3. Turgor pressure pushes solutes to sink, were it is needed while water moving in and out of the sieve tubes. 4. Removal of sucrose at sink increases water potential causing water to move out of the sieve tube at the sink. 5. Solutes move to sink cells and water goes back to xylem.
ATP and H+-carrier in the cell membrane are used to pump protons out of the sieve tube.
2. A proton gradient forms across the membrane with high H+ concentration on the outside of the membrane, K+ enters to keep charge balance.
3. Diffusion of proton back into the sieve tube, through ATP-ase, is coupled to a carrier and powers the transport of sugar into the sieve tube.

http://www2.mcdaniel.edu/
In this model, there is both passive and active transport. Passive: Long distance. Osmosis, water movement into and pushing solutes down the tube. Active: Short distance movement, loading and unloading the sugar.
ATP is important in this process because: - It increases Phloem loading; - Sugars are pumped into sieve tube against a concentration gradient: the concentration of sucrose in the chlorenchyma cell is typically 10-50 mM, while that in a sieve tube of a minor vein of a leaf may be as high as 1M.
This process of phloem loading increases pH in the sieve tubes to 8 and decreases pH out to 5.5, that is why K+ is used to maintain a balance.
Unloading: Unloading may occur symplastically or apoplastically. It may vary in different species.
Loading: Loading of sieve tubes from the cell walls requires energy which is derived indirectly by the proton gradient.
1. ATP and H+-carrier in the cell membrane are used to pump protons out of the sieve tube.
2. A proton gradient forms across the membrane with high H+ concentration on the outside of the membrane, K+ enters to keep charge balance.
3. Diffusion of proton back into the sieve tube, through ATP-ase, is coupled to a carrier and powers the transport of sugar into the sieve tube.
ATP is important in this process because: - It increases Phloem loading; - Sugars are pumped into sieve tube against a concentration gradient: the concentration of sucrose in the chlorenchyma cell is typically 10-50 mM, while that in a sieve tube of a minor vein of a leaf may be as high as 1M.
This process of phloem loading increases pH in the sieve tubes to 8 and decreases pH out to 5.5, that is why K+ is used to maintain a balance.
Unloading: Unloading may occur symplastically or apoplastically. It may varyin different species. http://www2.mcdaniel.edu/

Schematic diagram of transfer and transport processes contributing to the flow of assimilates acquired from aerial or soil environments, through the source-path-sink system. CO2 fixed by photosynthesis in chloroplasts gives rise to sucrose and starch. Sucrose, amino acids and mineral nutrients are loaded into sieve element—companion cell (se—cc) complexes of leaf phloem for long-distance transport to non-photosynthetic sinks. These solutes are exchanged reversibly between se-cc complexes and short- and long-term storage pools along the axial pathway. Short-term storage pools include phloem apoplasm, whereas the protoplasm of non-transport cells provides a long-term storage pool. In sink tissues, solutes are used for respiration, growth or storage.www.plantsinaction.science.uq.edu.au
As a result, massive hydrostatic pressure develops within the sieve tubes at the loading end, while the receiving end of the sieve tube micro pipeline assembly is kept under negative pressure because the organic solutes are constantly utilized by the active and expanding cells. Thus, a steep gradient develops between the supply end and the receiving end. This acts as the motive fore, where massive hydrostatic pressure developed at the supply end pushes the sucrose and other accompanied organic components enmass towards the sink. Munch demonstrated this phenomenon by an experiment.
ONWARD MOVEMENT BY ACTIVE TRANSLOCATION;
Once sieve tubes are subjected to massive hydrostatic pressures, the sucrose and other components that are loaded into sieve cells are virtually pushed forward along the concentration gradient. The initial motive force in this process is the hydrostatic pressure.

horticulturetalk.wordpress.com
The basic idea of how flow in the phloem works was described by Ernst Munch in 1930. The concept is a pressure-driven bulk flow model. To understand this, you have to realize that materials flowing from sieve tube element to sieve tube element do not go through any membranes…so the phloem is basically one cell, continuous from leaf to root and leaf to apical bud. A basic diagram here helps us to understand how this works.
As you can see, the cells of the leaf produce sugars that are loaded by transfer and/or companion cells into the phloem. The resulting reduced Ψs here lowers the water potential and water moves from the xylem into the phloem cell too. The combination of sugar and water moving into the phloem cell increases the pressure Ψp in the cell, pushing cytoplasm by bulk flow away from the leaf and toward the sink organs (root, etc.).
A pressure gradient is established along the phloem column from leaf to sink. The pressure is reduced along its length as sugar is taken out by adjacent cells and water follows osmotically. In the ultimate sink tissue, there is still sugar present to be unloaded and for water to follow. Thus, from source to sink, the pressure gradient is what provides the power for bulk flow. It is subject more to Poiseuille’s than to Fick’s factors.
Because the source is in the middle of the plant, with sinks at each end of the plant, the idea of phloem as a huge symplast with unloading at each end, and loading in the source area, explains the required bidirectional flow of solutes translocated in phloem. http://horticulturetalk.wordpress.com/

Comparison of source-to-sink sugar transport in symplastic and apoplastic active phloem loaders. Sucrose available for export from mesophyll cells (MC) results from a balance between storage in the vacuoles and sequestration as starch in the chloroplasts. Sucrose can reach the sieve tubes through plasmodesmata that allow for its diffusion from cell to cell in species like cucurbits. Sucrose is converted to larger molecules (RFOs) by the sequential addition of galactosyl residues in modified companion cells (CC) called intermediary cells. The larger molecules cannot move back to phloem parenchyma cells (PP) and are transferred and accumulated in sieve tubes. In apoplast-loading species, sucrose reaches phloem parenchyma cells through plasmodesmata. Sucrose is loaded and accumulates in the phloem by passing through the apoplast between the PP and the CC. The major players are presented in the enlargement of that area. Sucrose enters the apoplast through facilitators of the SWEET family (pale green circle) and is accumulated in the companion cell by a proton/sucrose co-transporter of the SUT1/SUC2 type (green circle). The energy necessary for the co-transport is provided by an H+/pumping ATPase (black circle) which establishes a proton gradient and a trans-membrane potential regulated by potassium channels of the AKT2/3 type (white circle). In Solanaceous species, SUT1 transporters are localized at the plasma membrane of sieve elements (not shown). Polyols can also be transported into the phloem, with specific transporters located in the plasma membrane of CC (not shown). A high hydrostatic pressure is generated in the sieve tubes of the collection phloem and water from the xylem is attracted. Sucrose, RFOs and polyols are transported in the sieve tubes to the sink organs in the transport phloem. All along the path, they can be leaked from and reloaded into the phloem via the same mechanism (not shown). Sucrose is unloaded into the release phloem where the hydrostatic pressure is supposed to be lower. Sucrose can be unloaded through a symplastic pathway or through an apoplastic pathway. In the latter case, sucrose is unloaded into the apoplast through specific carriers which can be of the SUT1/SUC2 type (green circle; Carpaneto et al., 2005). Sucrose is then taken up by sink-specific sucrose carriers of the same SUT1/SUC2 (light green circle) or converted to hexoses by a cell-wall invertase (CWInv). Hexoses are then taken up by specific carriers at the plasma membrane (orange circle) or at the tonoplast level (yellow and brown circles). Sucrose in sink cells can be metabolized (growing sinks) or stored as starch in amyloplasts, or imported into the vacuoles (red circles) and further converted to hexoses by a vacuolar invertase (VInv). http://journal.frontiersin.org/
In spite of the development of mass hydrostatic pressure at the supply end, it does not explain the rate of diffusion of organic solutes in such massive quantities, because the rate of translocation of solutes reaches an astounding rate of 55-5000 cm/hr; which is actually 10,000 to 20,000 times faster than the recorded rates of diffusion, furthermore, the translocation is an active process that is ATP dependent.
This impasse has been explained by involving P-proteins which run from one sieve cell to another as inter connecting active tubular system. The P Proteins consisting microtubules, microtrabaculae and actin-myosin elements actively transport organic solutes, preferably sucrose along the surface of microtubular elements. In fact, the organic solutes are virtually pushed or glided over the surfaces of tubular microtubules by the activity of kinesin like protein activity. The process is active because it expends ATP energy. The required energy is supplied by mitochondria that are present in sieve tube cells per se; but most of the energy rich molecules are supplied by associated companion cells. Furthermore, the flow of organic solutes all along the surface of phloem microtubular elements is so smooth, it is never impeded by the presence of sieve plates for the tubules are continuous and they run from one end to the other. In fact, such activated transport can reach a velocity of 55-500 cm/hr which is actually an observed fact.
EVIDENCES TO SUPPORT THE ABOVE SAID MECHANISM;
The above said events as a mechanism of transportation of organic solutes are supported by the following facts:
1. The presence of longitudinally oriented P-protein having microtubules, microfilaments and microtrabaculae has been demonstrated by high voltage electron microscopy. Such protein complexes are found across the sieve tubes running from one sieve tube to the other.
2. In vitro polymerization of tubulin monomers into tubular microtubules has been perfecting. Using invitro microtubules, it has been shown that micro tubules spread on a glass plates perform the movement of partientated on their outer surfaces.
3. The mass pressure developed in sieve tubes present in leaves shows greater positive pressures. In fact, the estimation of sucrose content of sieve tubes at the vein loading site is about 20-40% which is equivalent of + 20-40 atmospheric pressure.
4. The entire process is energy dependent, for the respiratory inhibitors like KEN, DNP, Rotenone, etc; readily inhibit the active transportation of organic compounds to a greater extent. Still the diffusion of organic solutes continues along with the concentration gradient, which when compared to the normal process is many thousand times slower. Use of radioactive tracer elements like 35 S-Methionine, has shown that though the sieve tube cells are enucleated, the cells are active in the synthesis and polymerization of P-Proteins and its associated components. Such proteins exhibit rapid turnover during the transportation of organic solutes.
5. Colchicine treatment completely abolishes the transport of sucrose similarly most of the sieve tubes under cold conditions prevent polymerization of tubulin into microtubules, so hinder the movement.
PROTOPLASMIC THEORY ;
Protoplasmic theory is based on the observation of protoplasmic streaming in other plant cells, where the protoplasm exhibits rapid circulatory movements in which it carries some visible small granular structures. As sieve tubes being living cells, DeVries has assumed that the solutes are transported along the protoplasmic stream from one cell to the other across the sieve plates. If the observed rate of protoplasmic streaming is compared to the actual rate of translocation, it is slower at least by 1000 times. This process does not implicate the requirement of mass flow or pressure flow.
TRANSCELLULAR STRAND THEORY :

Sieve Tubular cell’s Transcellular strands.
Transcellular strand theory was proposed by Thiane et al. Al. According to them certain protoplasmic strands are found within the sieve tube which traverses the sieve plates. Organic solutes are assumed to move along these strands. But such defined transcellular strands have never been observed by others. But, recent electron microscopic studies reveal the presence of a large number of large numbers of P-proteins which run parallel to each other, across the sieve plates. Visualization of such strands by these people without electron microscopic studies is highly creditable. Full credit should be given to their concept of transcellular strands as means of translocation of organic solutes.
CONTRACTILE PROTEIN THEORY :
Observations by Tension and Williams about the presence of a network of micro fibrils in sieve tubes and the involvement of such contractile proteins in the transport of solutes, though reported other, plant physiologists were skeptical about them. It was very unfortunate for it is now known that such contractile P-proteins with their associated microtrabacular network are found within sieve tubes, is a fact. And they are mainly responsible for the translocation.
Munich's "Pressure Flow" model (1927):
Mass or pressure flow hypothesis proposed by Munch has enjoyed wider acceptance. The general agreement with this view is due to certain experimental evidences. A membranous bag a filled with a lot of sucrose or other organic compounds and another membranous bag B-filled with just water are interconnected with a narrow-bent glass tube.
The bags are immersed in two interconnected troughs filled with water. As soon as the bag A is immersed in water, water from the trough rushes into the membranous bag A and push the liquid from A towards B for bag A is filled with water only.
Munich's model best describes the movement in the phloem. His model suggests that there is a turgor-pressure gradient that drives the directional mass flow of the solutes and water through sieve tubes of the phloem.

This can be demonstrated with an osmometer permeable only to water filled with a high concentration of solutes in one arm as shown below. When the osmometer is put in distilled water, the water potential is less than that of surrounding water in the one arm, and water will enter by osmosis which the generates a turgor pressure, The solutes are carried by bulk flow when the water moves in. Eventually this process will stop when the pressure throughout equalizes. http://www2.mcdaniel.edu/

Sieve Tubular cell’s Transcellular strands.
The flow of Leaves synthetic mass materials has to be transported to fruits, seeds and many such structures, for which sieve tubes require well organized protein tubular structures for efficient flow of the materials to the respective structures- such as fruits- variety of them, for example Tomato, mango, coconut and many similar structures sieves require a well-organized protein tubules for it and leaves are the source for it for only leaves can produce such components.

