Lipid Metabolism
Lipids are a kind of hydrocarbons which are insoluble in water but soluble in organic solvents like chloroform, acetone and other similar solvents. Lipids are esters of fatty acids and alcohol derivatives or forms like glycerol. They have some important functions to perform such as:
1. They act as very essential structural and functional components of cellular membranes.
2. They act as storage or mobile metabolic fuels.
3. They provide waxy coatings on the surface of plants and animals.
4. Some of them act as important vitamins and hormones
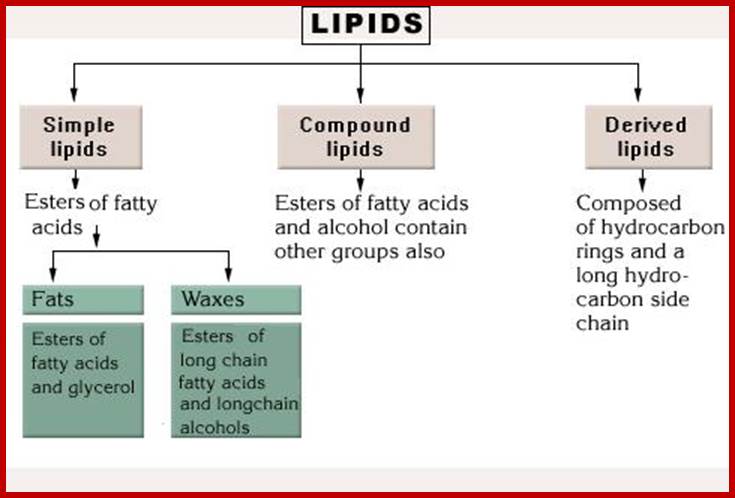
Based on the chemical composition and properties, lipids have been classified into simple lipids and compound lipids.
Simple lipids
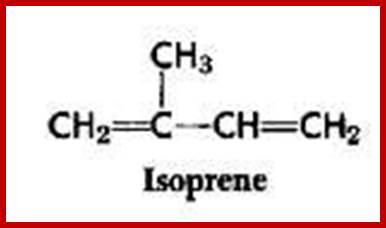
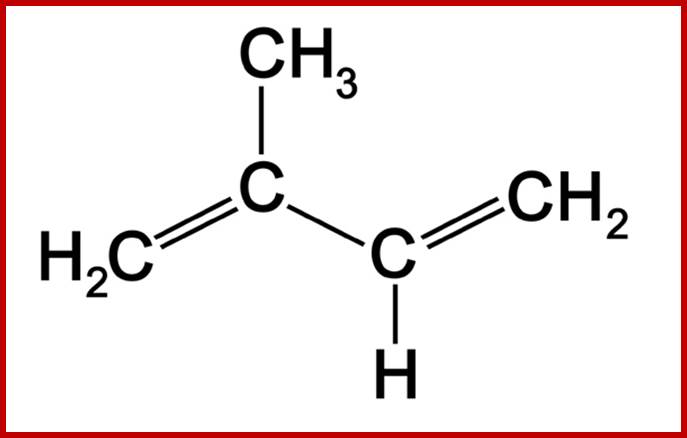
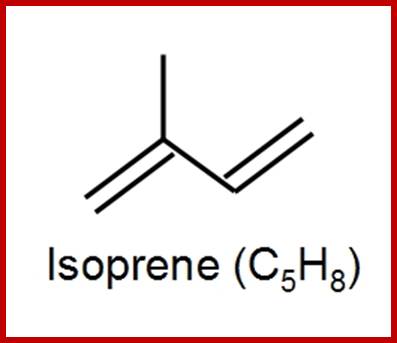
Simple lipids are also called as nonsaponifiable lipids because they do not possess any true fatty acids or its derivatives. Living cells contain small amounts of this kind of lipids. But certain forms of these lipids play significant roles in life activities. Ex. Vitamins, hormones, etc. The non-Saponifiable lipids have been grouped into two classes. They are terpenes and sterols and both of them are derived from the same common five carbon building blocks called isoprene units. The isoprene unit is a 5 carbon 2 methyl 1.3 butadiene compounds. Such isoprene units join to each other tail to tail or head to tail. Isoprene derived Terpenes- monoterpenes 2 units; sesquiterpenes-3 units; diterpenes- 4 units; sestertepenes-5 units; Triterpenes- 6 units, Carotinoids-8 units and rubber>100 units.
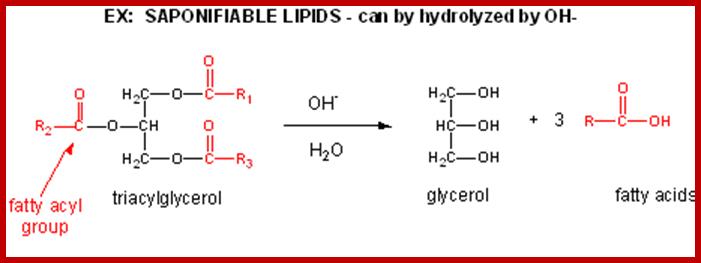
Saponifiable and non-saponifiable lipids, based on their reactivity with strong bases: Saponifiable lipids contain long chain carboxylic (of fatty) acids that are linked to an alcoholic functional group through an ester linkage. These fatty acids are released on based catalyzed ester hydrolysis. The non-saponifiable classes include the "fat-soluble" vitamins (A, E) and cholesterol. Lipids are often distinguished from another commonly used word, fats. Some define fats as lipids that contain fatty acids that are esterified to glycerol. I will use the lipid and fat synonymously http://employees.csbsju.edu/.
Terpenes:
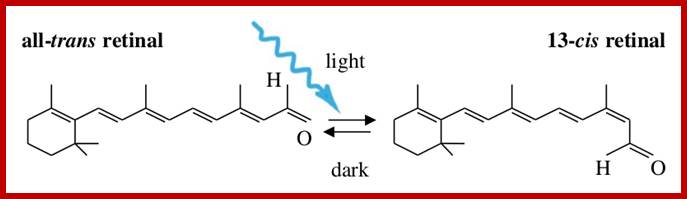
They are made up of multiple units of isoterpenes or isoprenes. If two isoprene units are joined to each other, such terpenes are called monoterpenes (10C). And those with three, four, six to eight isoprene units are called sesquiterpene (15C), diterpenes (20.C), triterpenes (30G) and tetraterpenes (40C) respectively. Because of the presence of double bonds in linear terpenes, they often assume trans or cis configuration. Among a variety of terpenes found in plant parts, B-carotene, vit E, vit K play important roles in cellular metabolism. When B carotenes split in the middle, they yield retinols called vit-A. The splitting of beta carotenes into Vit-A’s takes place only in animal intestines. They are the important components of retinols required for eye sight.
http://cronodon.com/NatureTech
STEROIDS:
www.cyberlipid.org; http://cronodon.com/NatureTech
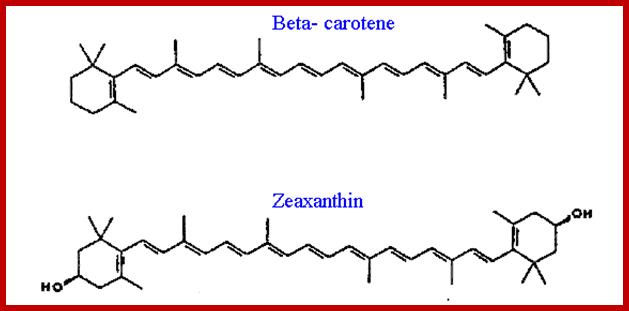
Beta-Carotene and Zeaxanthin; www.biolozi2012.files.wordpress.com
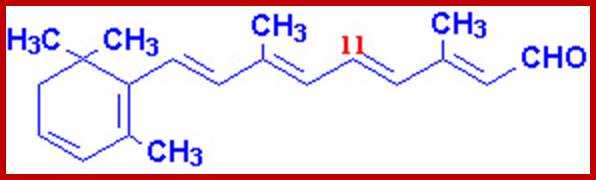
Trans Retinol ((vitamin-A); www.ch.ic.ac.uk
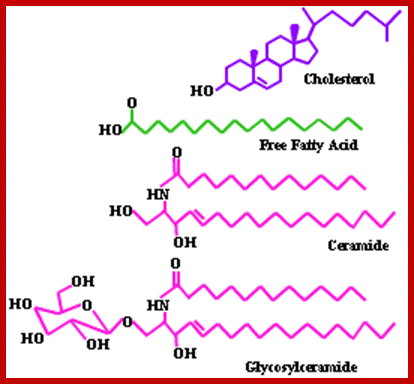
Well characterized steroids:
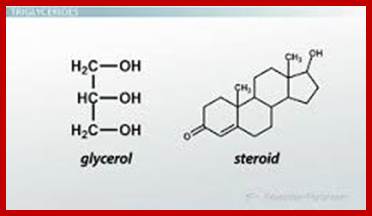
They are the derivatives of saturated tetracyclic hydrocarbons. A great number of steroids have been extracted and identified from plant sources. And the structures of them have been determined. Many steroids have been found to play specific functions in cellular structures and activities. Cholesterol, Cholic acid, Lanosterol, Testosterone, β-Estradiol, etc. are the common sterols found in plants.
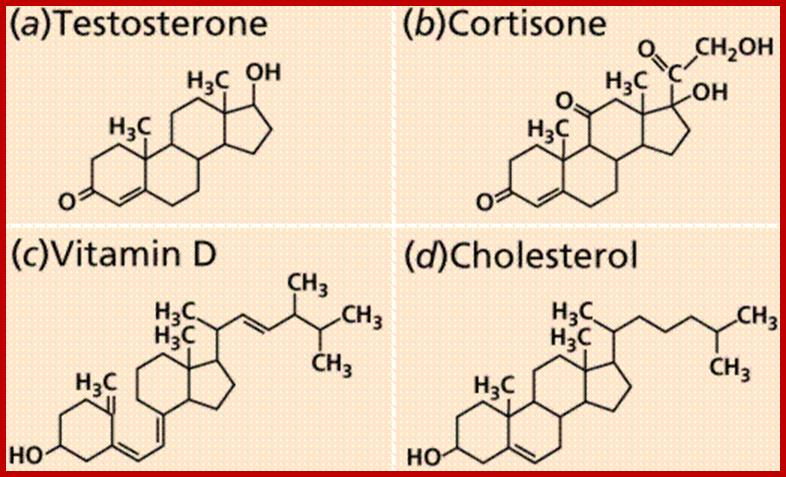
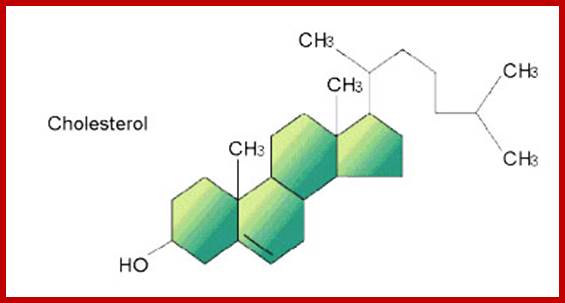
Cholesterol
ESSENTIAL OILS
Various plants, depending upon the species, produce volatile oils which exhibit different but characteristic odors. Some of the essential oils yielding plants have been commercially exploited ex., peppermint, sandal, rose, lavender, geranial, turpentine, lemon, etc. Most of these terpenes are metabolic byproducts of plants.
RUBBER
Rubber is another isoprene derivative consisting of 500-5000 isoprene units per molecule of rubber. A large number of plants produce latex which is rich in these compounds. The same is commercially exploited in the manufacture of various kinds of rubber and rubber products.

http://www.chm.bris.ac.uk/
COMPOUND LIPIDS
Saponifiable lipids are also called compound lipids. They are actually esters of fatty acids and alcohol like glycerols. On alkaline hydrolysis, they produce salts of fatty acids called soaps. Depending upon the kind of alcohol or alcohol derivatives and the kind of fatty acids that go into esterification, different kinds of compound lipids can be identified. However some of them remain in liquid forms at room temperature and they are called oils. This is because the linear chains of fatty acids contain a number of unsaturated double bonds, but there are others which exist as solids at room temperature because the fatty acids contain saturated carbon to carbon linkages.
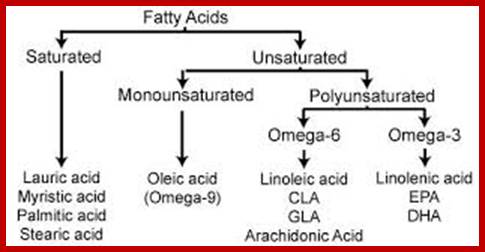
List of Few–Saturated and unsaturated Fatty Acids:
Propionic acid (3C:0)
Butyric acid (4C:0)
Lauric (12C:0)
Myristic (14C:0)
Palmitic (16C:0)
Stearic (18C:0)
Arachidic acid (20C:0)
Hexatriacotylic acid (36C:0)
Alpha-linoleic acid (18C:3)
Stearidonic acid (18:4)
Linoleic acid (18C:2)
Linoleic acid (gamma) (18C:3)
Oleic acid (18C:1)
Stearic, Palmitic and oleic acids are the common fatty acids found in plants and animals. But plants in general contain greater amount of unsaturated
Lipids = Fatty acids and Glycerols
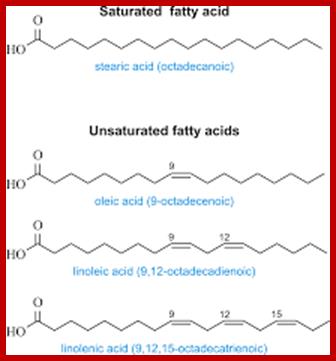
Some common fatty acids; www.periodni.com
Fatty acids with double bond between certain carbon atoms, which gives the unsaturation for the chain and they remain in liquid (oil or fluid) state under normal temperature.
Classification of phospholipids and synthesis of Phospholipids
www.etd.ohiolink.edu
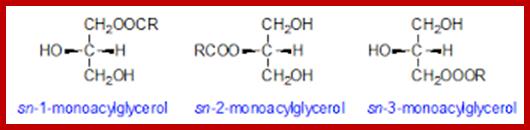
Glycerol consists of three OH groups and each one of these can be esterified with fatty acids of the same kind or of different kinds. In many cases, one of the OH groups is linked with compounds other than fatty acids. The combinations of both can yield a variety of mono, di or triglycerides; the melting point of the above said glycerides depends upon the kind and the length of fatty acid chain. Such glycerides can be subjected to saponifications.
Glycerol and steroid; education-www.portal.com
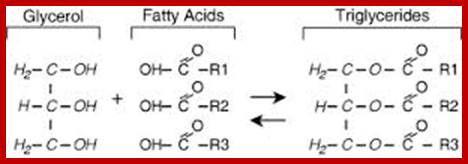
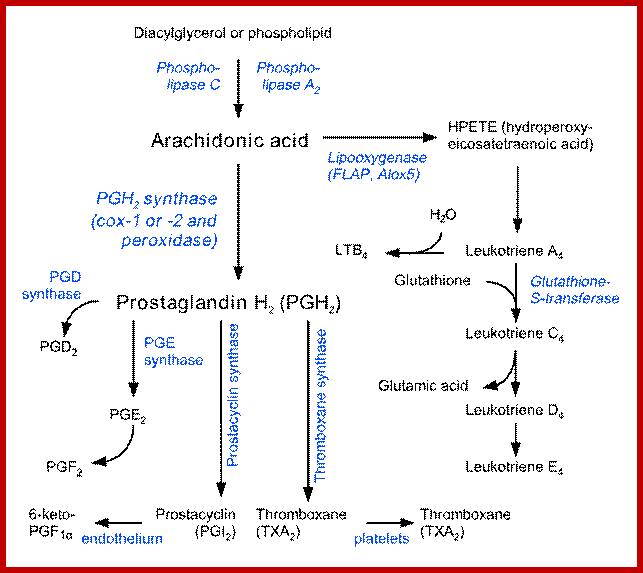
The following are the diagrams of some fatty acids, lipids, prostaglandins and their relative components. Fatty acids linked to glycerol
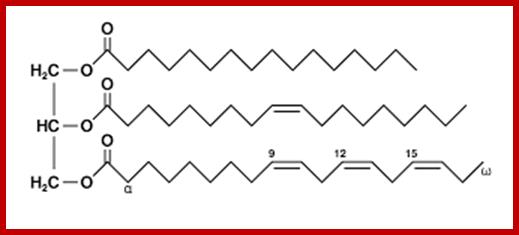
Triglyceride-covalently bound Fatty acids to Glycerols; www.alevelnotes.com
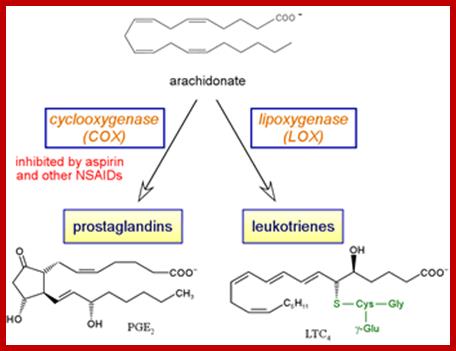
Intervonversions of Arachidonate;www. guweb2.gonzaga.edu
Breakdown of triglycerides by specific enzymes; www.biochem4.biochem.okstate.edu
Tri-acylglycerol; www.reducetriglycerides.com
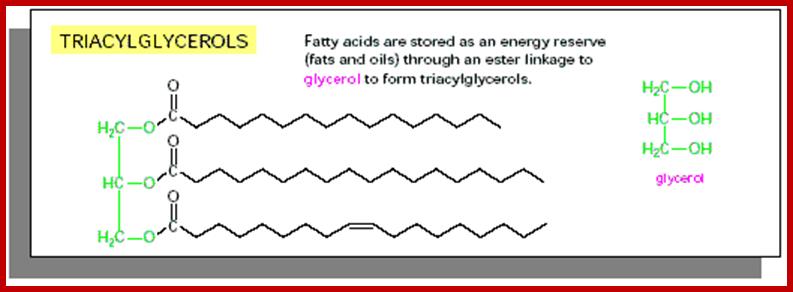
Triacylglycerol (fat Molecule); www.galilu.com

Phosphatidate. www.imgarcade.com
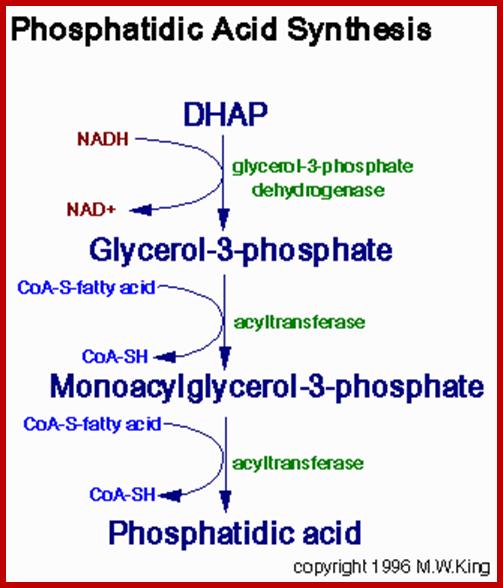
Phosphatidic acid synthesis www.rclace.eu
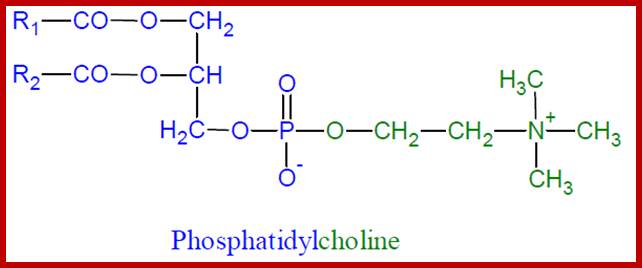
Phosphatidylcholine; www.nootropicsupplementreview.com
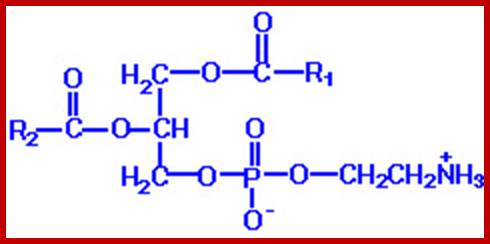
Phophatidylethanolamine. http://www.guidechem.com/
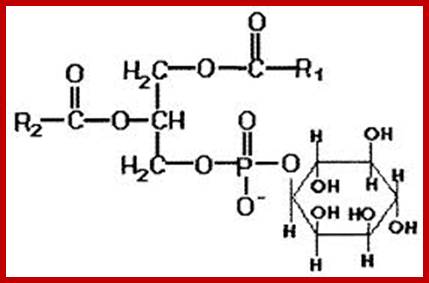
www.imgarcade.com; Phosphatidylinositol
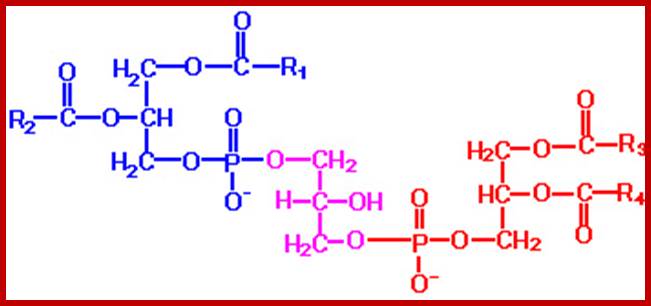
Cardiolipin; www.homepage.smc.edu
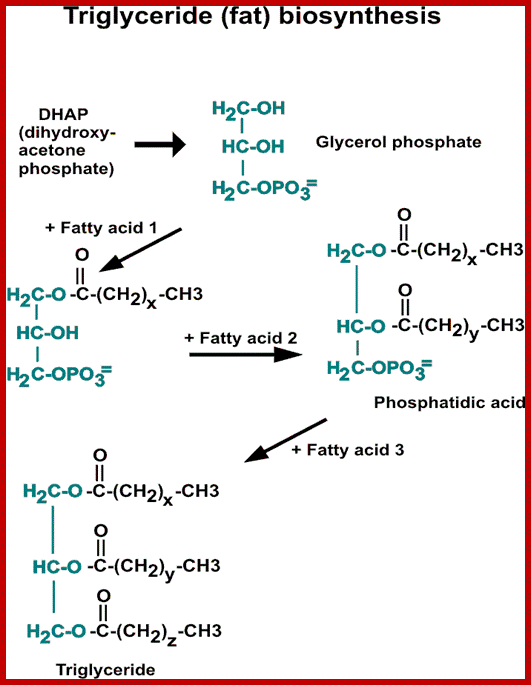

Liid synthesis in soybeans; www.imgarcade.com
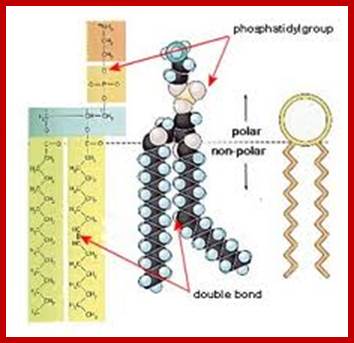
The acyl glycerols may exhibit further modifications. For example, in di acyl glycerols, the 3rd free OH group may be linked with carbohydrates, phosphates, sulphates proteins etc. Accordingly, various kinds of acyl glycerols can be recognized. Alkyl glycerol, acyl glycerol, glyconyl acyl glycerols, phosphoacyl glycerols, sphingolipids, etc., are few examples of modified acyl glycerols. Plants contain most of the above said lipids, but the most common and abundant form of lipids found is glycolipids and phospholipids. Particularly, phospholipids exhibit bipolarity with hydrophobic fatty acid chain as the tail and hydrophilic phosphate group as the head. Some of the common phospholipids found in plant cells are phosphotidyl ethylamine, phosphotidyl choline, phosphotidyl inositol, and cardiolpin and phosphotidyl serine. The net polar charges of the above phospholipids vary from pH to pH. Phospholipids can be easily separated and identified by thin layer chromatographic methods.
Glycerol phosphate produced by glycerol 3 phosphate dehydrogenase or by glycerol kinase, acts as an acceptor of fatty acyl CoA chains. The enzyme glycerophosphate acyl transferase brings about the acylation reaction between OH group of phosphoglycerate and fatty acid Co.A to produce mono or di acyl glycerols. Specific modifications are performed by specific enzymes.
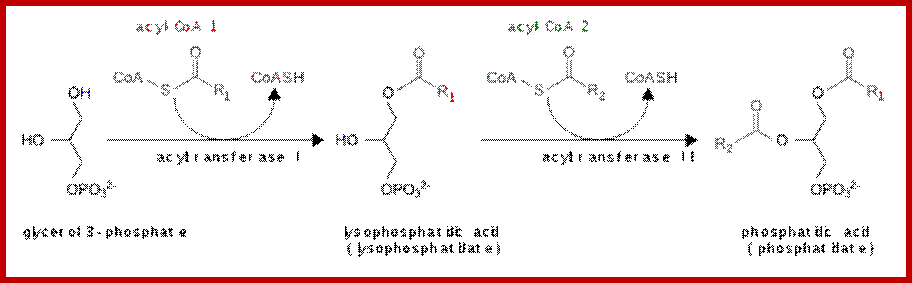
Glycerol 3-phosphate to phosphatides. www. en.wikipedia.org
HYDROLYSIS OF PHOSPHOLIPIDS
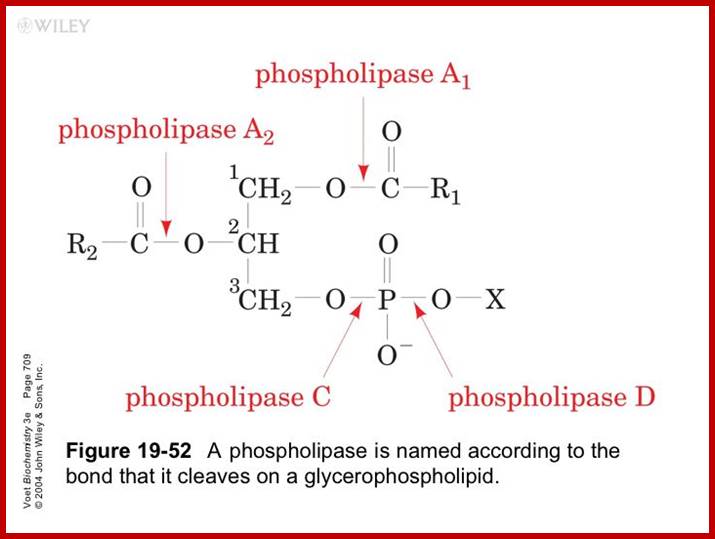
Phosphoglycerides are hydrolyzed by specific enzymes called phospholipases as shown in the. Phospholipases-A cleaves the fatty acid at the 1st position (A). Phospholipase-B, at 2nd position (B), Phospholipase C at third position (C) where it cleaves the bond between 3rd carbon of the glycerol and phosphate. On the other hand, Phospholipase-D removes the polar group at a position-D.
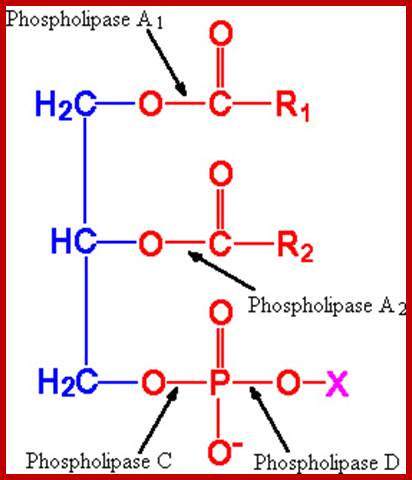
Action of Phosphoiipases. www.agridr.in
WAXES
Waxes are the esters of higher fatty acids with long chain monohydroxy fatty acid alcohols such as acetyl alcohol, ceryl alcohol and mericyl alcohol. Waxes are insoluble esters and remain as solids at room temperature but they are pliable at little higher temperature. Waxes along with cutin are secreted and deposited by the epidermal cells as surface coatings and such layers protect plant body and also they prevent loss of water from the surface of the plant body.
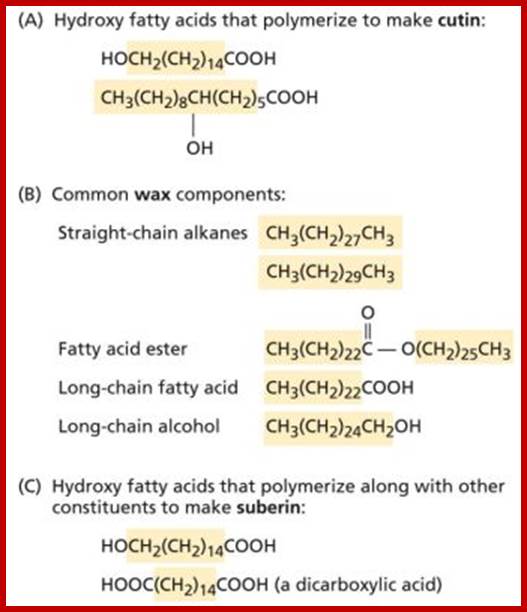
CUTIN
Cutin is primarily made up of fatty acid esters but they also contain fatty acids. Again, as mentioned above, cutin is secreted by epidermal cells which will be deposited in layers fatty acid esters. The cuticle also contains waxes. But cutin accounts for 50-60% of the cuticle. The extent of deposition depends upon the environmental factors and the inherent genetic factors. The cuticle, apart from protecting the surface of the plant, it also protects the loss of water from the plant surfaces as in the case of waxes. On the contrary, aquatic plants do not produce any cuticle layers.

Plants use a hydrophobic cuticle made of cutin and waxes to protect themselves from the environment. Investigation of a tomato mutant deficient in cutin biosynthesis now reveals the first cutin synthase, capable of converting monomeric hydroxyacyl chains into polyesters. This image shows the cuticle (red) and polysaccharide cell wall (blue) of an M82 tomato cultivar. Cover art by Erin Dewalt, based on an image from Gregory Buda. Brief Communication, p609; News & Views, p603; www.nature.com
Cuticle is composed of waxws and polymer network of large molecular fatty acids as building blocks called Cuticle;www.news.cornel.edu
SUBERIN
It is an ester of glycerol and phenolic compounds. Thus, suberin is quite different from cutin. Suberin is deposited in cell walls of phellum or bark during secondary growth. Suberin also shows a property of impermeability to water.
5e.plantphys.net
www.plantphys.info

http://www.bio.miami.edu
Suberin is a polymer whose structure is poorly understood. Like cutin, suberin is formed from hydroxy or epoxy fatty acids joined by ester linkages. However, suberin differs from cutin in that it has dicarboxylic acids (see Web Figure 13.1.A, bottom), more long-chain components, and a significant proportion of phenolic compounds as part of its structure.
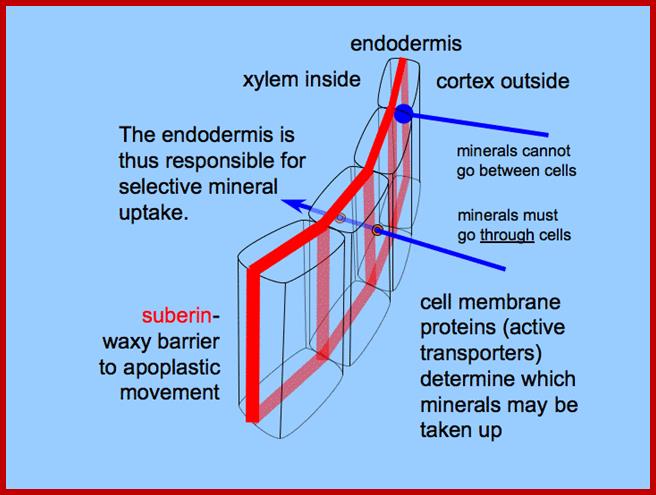
Suberin is a cell wall constituent found in many locations throughout the plant. We have already noted its presence in the Casparian strip of the root endodermis, which forms a barrier between the apoplast of the cortex and the stele (see textbook Chapter 4). Suberin is a principal component of the outer cell walls of all underground organs and is associated with the cork cells of the periderm, the tissue that forms the outer bark of stems and roots during secondary growth of woody plants. Suberin also forms at sites of leaf abscission and in areas damaged by disease or wounding. It is a special type of wax,
Suberin is important especially in the cells of the outermost layer of woody plant bark, known as cork. All plants that produce true, botanical wood produce a layer of cork, which is largely impervious to water and gases. (The cork of the Cork Oak, Quercus suber, is used to make wine corks for this very reason; it's from this plant that Suberin gets its name.). Quercus suber’s cork is used to make wine corks
SYNTHESIS OF FATTY ACIDS
Plants as well as animal cells synthesize and degrade fatty acids all the time. But in plants, the synthesis of fatty acid is very much accelerated during the development of seeds and the same is stored in the seeds of peanuts, castor, beans, soybean, etc. In coconut plants, during the development of fruit the liquid endosperm gets transformed into solid cellular endospermous tissue. It is at this juncture, the genetic material gets activated to produce a set of mRNA, which on translation produces enzymes necessary for the synthesis of fatty acids and lipids in large amounts. Probably, this is one of the best examples, where the cellular machinery is fully activated for the synthesis of oils and fats on massive scale.
Synthesis of acetoacetyl-ACP from CoA.ACP is Acyl carrier protein; http://lecturer.ukdw.ac.id/
Elongation cycle- http://lecturer.ukdw.ac.id/
The synthesis of fatty acids occurs mostly in cytosol, outside the mitochondria. In plants chloroplasts are main site for the synthesis of fatty acids, which are then exported to cytosol. Citrate is very essential for the maximal activity of fatty acid synthesizing enzymes. Strongly, CO2 is also required for the initial reaction. The enzymes responsible for the synthesis of fatty acids are aggregated into multiple enzyme complexes. Fatty acid syntheses is a multistep process, where the intermediate products remain bound to the enzymatic surface and only the final product is released from the surface of multiple enzyme complex. This enzyme complex is made up of seven proteins with a total mol. wt. of about 2.3 million Daltons.
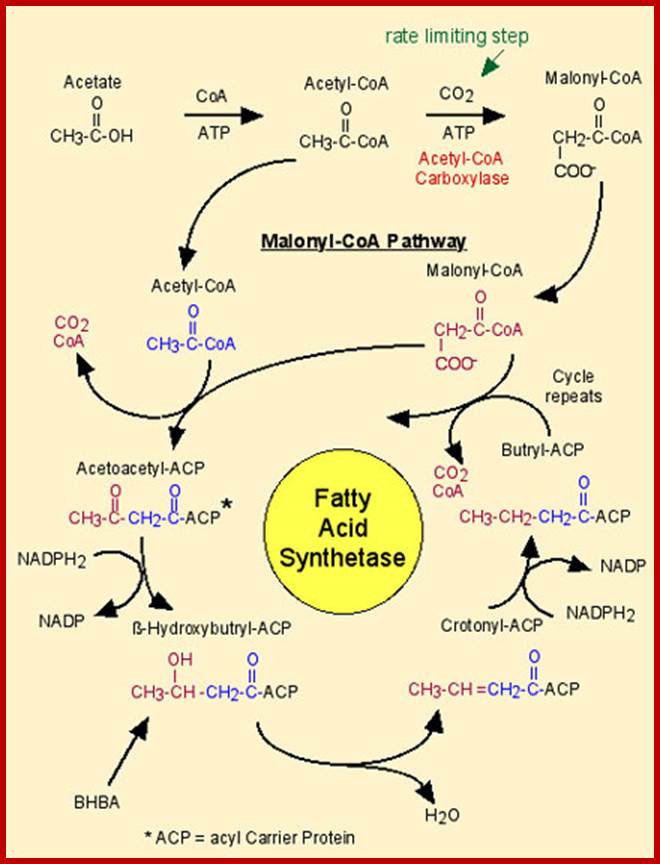
The main carbon source for fatty acid synthesis ia acetyl Co.A, which is produced in mitochondria by the oxidation of pyruvates, amino acids of by B oxidation of fatty acids. Acetyl Co.A is transported across the mitochondrial membranes felicitated by a carrier called carnitine protein. For example, one palmitic acid (16C) is synthesized by condensation of one acetyl Co.A (2C) and seven malonyl CoA (2C) seven successive steps. In this process seven CO2 are releases. The reductive power is NADPH2 which is generated and supplied HMP pathway.
SYNTHESIS OF MALANYL CO.A s
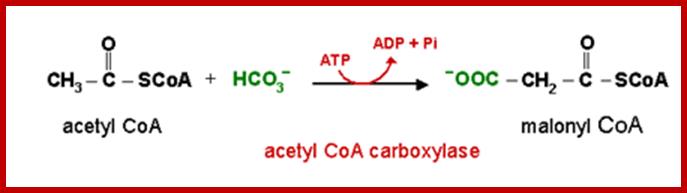
Initially, the enzyme acetyl Co.A carboxylase, utilizing ATP as the energy source, condenses 1 acetyl Co.A with 1HCO3 to produce one malonyl Co.A. The enzyme requires biotin as the coenzyme.
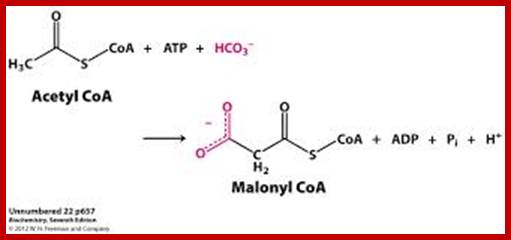
www.oregonstate.edu
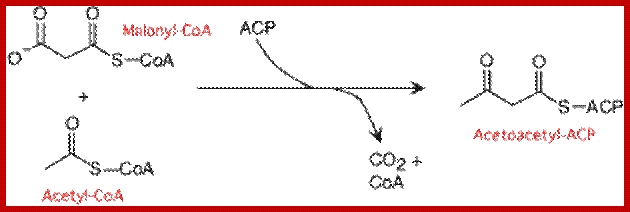
Synthesis of Acetoacetyl ACP from CoA; lecturer.ukdw.ac.id
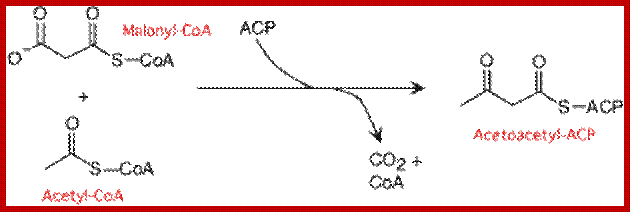
Once malonyl Co.As are made available, fatty acid synthesis proceeds in six successive condensation cyclic steps. At the seventh cycle, ACP protein Acyl carrier protein binds covalently to the fatty acid chain.
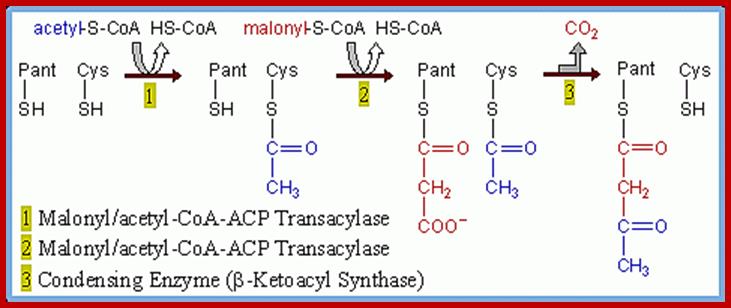
PRIMING STEPL:
In this step, acetyl Co.A binds to ACP protein. This reaction is brought abut by ACP acyl transferase. Then the acetyl group bound to ACP protein is immediately transferred to the second enzyme of multienzyme complex called b-ketoacyl ACP synthetase.
ADDITION OF MALONYL Co.A
This process takes place in two steps. In the first part of the reaction, malonyl S Co.A is transferred to ACP SH moiety. In this reaction Co.A-SH is released. The enzyme involved in this reaction is ACP malonyl transferase.
At this stage of the reaction, one malonyl group is esterified to ACP complex and one acetyl group is esterified to SH group of B-ketoacyl ACP synthetase of multienzyme complex. Once these two are brought onto the surface and positioned adjacent to each other, the B ketoacyl ACP synthetase brings about the transfer of acetyl group to the second carbon of the malonyl group which is still bound to the ACP malonyl transferase enzyme. Due to this transfer, the surface of B ketoacyl ACP synthetase enzyme becomes free. The condensation product is now called aceto acetyl S-ACP.
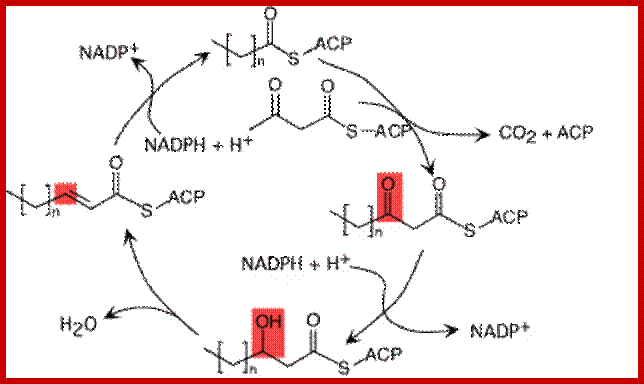
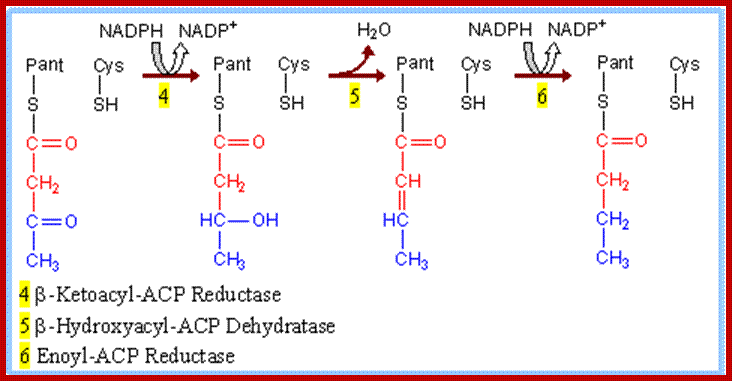
REDUCTION REACTION – STEP I
The aceto acetyl S-ACP is then subjected to reduction by the enzyme B ketoacyl ACP reductase. The product of this reaction is B-hydroxy butyryl-S-ACP, the synthetase enzyme becomes free.
DEHYDROGENATION REACTION
Then the B hydroxyl butyryl S-ACP is dehydrated by an enzyme called enoyl ACP hydratase. The product of this reaction is crotonyl S ACP.
Reduction Reaction Step II
Crotonyl S-ACP is further reduced to butyryl S-ACP by the action of an enzyme called enoyl ACP reductase. In this reaction, the hydrogen donor is not NADH2 but NADPH2.
Synthesis of butyryl S-ATP is the end of the first of seven cyclic reactions. In the second cycle, the butyryl group is transferred to synthase, so that ACP can receive another malonyl molecule. This is very essential for the next step of condensation, reduction, and hydration and reduction steps, for the growth of the fatty acid chain. This cyclic process is repeated seven times to produce 16 carbon palmitoyl S-ACP, which inturn is subjected to thioesterase reactions to yield free palmitic acid or palmitoyl S-ACP can be transferred from ACP complex to Co.A or it may directly be incorporated into phospholipids.
Generally, the multiple enzyme systems that are involved in fatty acid synthesis steps at 16C palmitic acid stops; further elongation of chain is performed by the addition of acetyl or malonyl groups through another set of ACP carrier proteins.
But the synthesis of odd numbered fatty acid chains requires the priming reaction with proponyl S-ACP instead of acetyl-S-ACP, to which 2 carbon units are added successively. Thus, the synthesis of fatty acids is a complex process and it is highly regulated. In animals, when excess of food material is available than that is needed, the same is converted and stored in the form of fatty acid ketone bodies. But in plants, in certain species fatty acids are stored in the form of oil globules in seeds and fruits, where they act as reserve food materials, ex. Peanuts, coconut, etc.

http://biosiva.50webs.org/
Fatty acid synthesis, www.ansci.illinois.edu
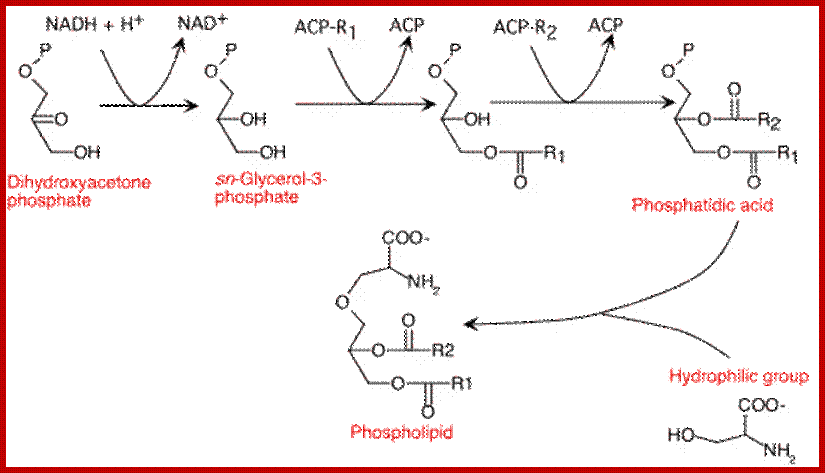
Lipid assembly; http://lecturer.ukdw.ac.id/
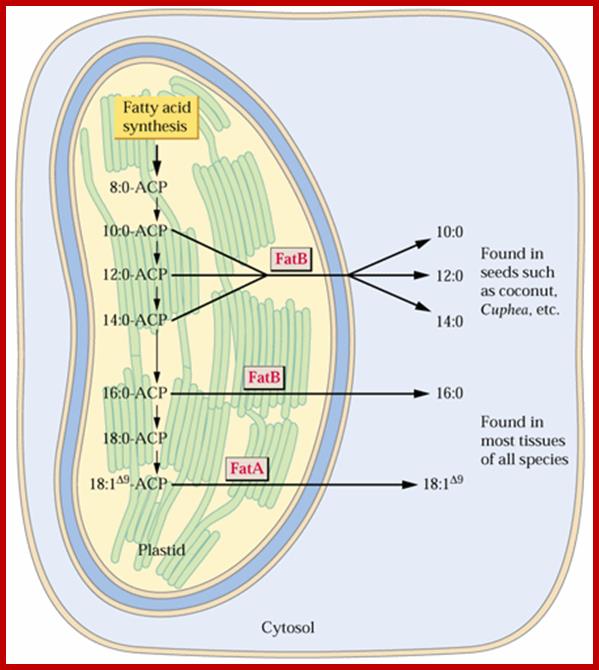
The fatty acids transferred to the cytoplasm enter the acyl-CoA pool providing fatty acids for glycerolipid synthesis (see lecture 15) or further elongation in the cytosol. The further elongated fatty acids (C20 to ~C32), known as very long chain fatty acids (VLCFAs), can occasionally be put into triacylglycerol or used in synthesis of wax, cutin or suberin.;Role of chloroplasts in fatty acid synthesis; www.uky.edu
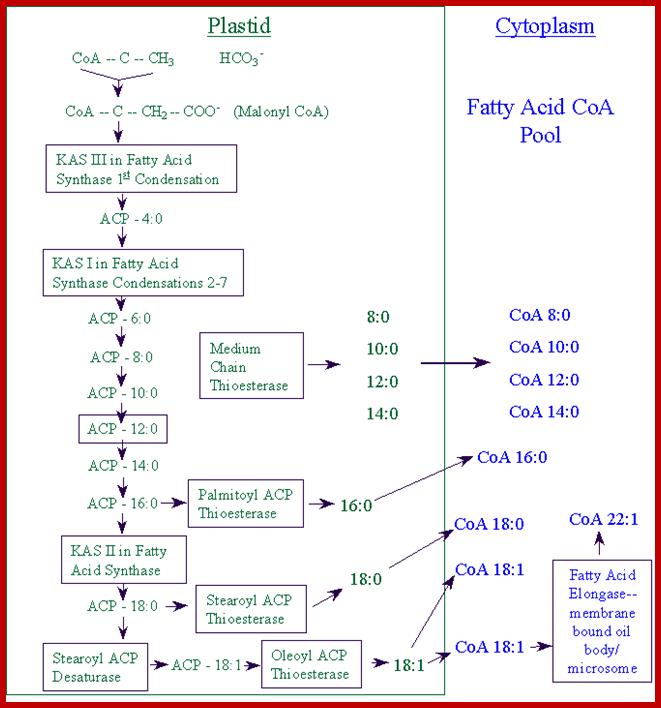
Saturated Fatty Acid Thioesterase genes are known as FatBs and that for the predominate unsaturated fatty acid-ACP synthesized in plastids FatA;The answer appears to be in the action of diesterase (TEs) which hydrolyze the acyl-S-ACP thioester bonds. In other words TEs stop the reaction at the appropriate chain length. Some plants have unusual TEs, known as medium chain TEs, which stop the reaction at C8, C10, C12 or C14 fatty acid chain lengths. Once a TE releases the fatty acids, they are either incorporated into plastid glycerolipids by acyltransferases (ATs) or they are transferred to the cytosol and esterified to CoA. This is illustrated in the following figure:;Plastid the site of FA synthesis; www.uky.edu
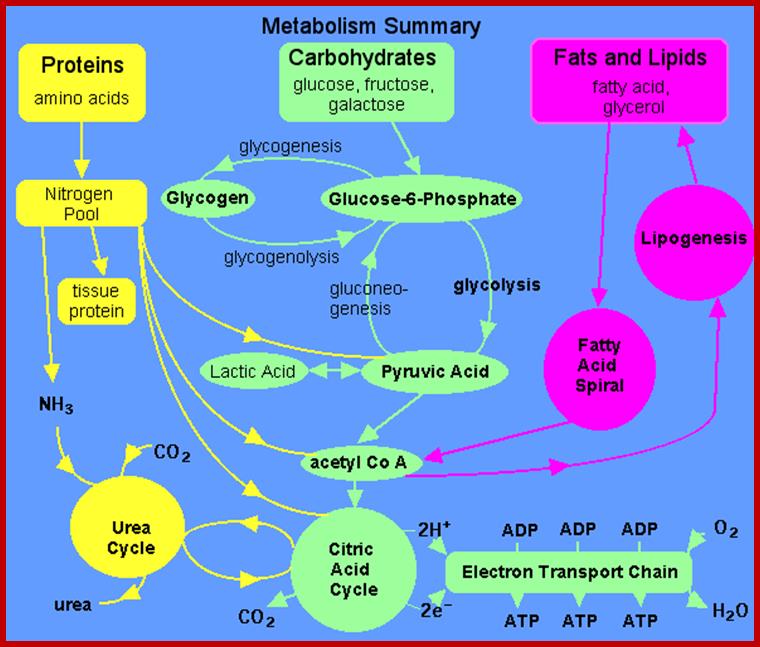
Metabolism is the sum total of all chemical reactions involved in maintaining the living state of the cells, and thus the organism. In general metabolism may be divided into two categories: catabolism or the break down of molecules to obtain energy; and anabolism or the synthesis of all compounds needed by the cells (examples are DNA, RNA, an protein synthesis). The diagram on the left contains a summary of all the types of metabolism that will be examined. In this module, the electron transport chain is examined. www.elmhurst.edu
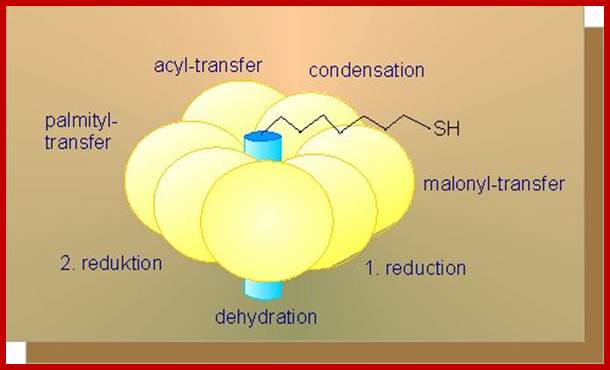
www.images.1233.tw
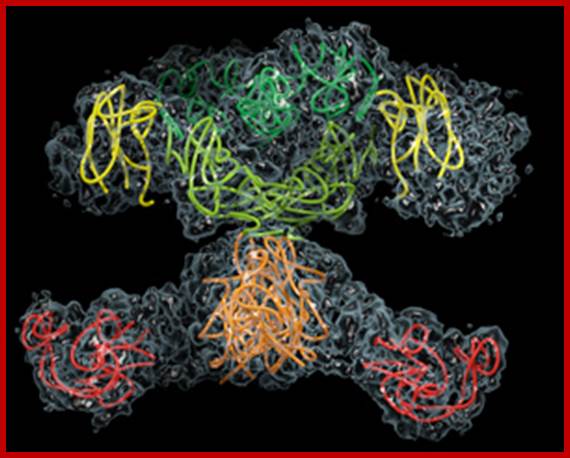
Not an X-chromosome, but the structure of the fatty acid synthase complex of mammals. Individual enzymes are coloured, the matrix structure is grey. ;The structure of fatty acid synthase of fungi resembles a barrel. Individual enzymes are coloured, the matrix structure is grey. (Picture: Simon Jenni and Marc Leibundgut) large; http://archiv.ethlife.ethz.ch/

OXIDATION OF LIPIDS
Lipids stored in the plant organs like seeds and fruits are energy rich components and the same are used at the time of germination of seeds by oxidative process which is quite different from the oxidation of carbohydrates or proteins.
Though plants and animals store lipids in various forms, the basic mechanism of oxidation of lipids is virtually the same. For example, if the stored lipid is phosphotidyl choline, specific phospholipases act upon them and remove the fatty acid chains from glycerol step wisely. The action of phospholipase A, B, C & D, has been very specific and the same has been described in the text elsewhere. However, to remind the readers about the mechanisms, it is known that phospholipase A breaks the ester bond at the first carbon position to yield isophosphoglyceride. Phospholipase B-hydrolyses the ester bond, at second carbon position to yield lysophosphotidyl ethalomine. Similarly, phospholipase C breaks the bond between the third carbon bond of glycerol and phosphate group, but phospholipase D breaks the bond between polar head group and phosphate at the 3rd carbon of the glycerol to yield phosphotidic acid intact.
Glycerols thus liberated are drawn into respiratory metabolic reactions, but the free fatty acids are subjected to different types of oxidation reactions which are quite distinct and characteristic from glucose oxidation reactions. In fact, there are two basic mechanisms by which h fatty acids are oxidized, namely Alfa oxidation and B oxidation, where fatty acid chains are systematically but sequentially cleaved into two carbon energy rich compounds. Plants have the ability to perform both kinds of oxidations efficiently.
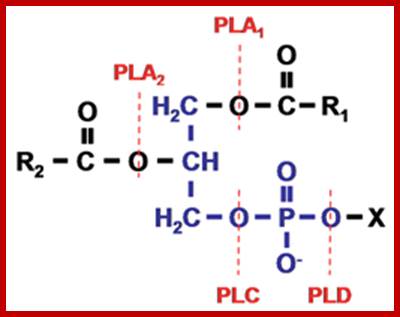
Phospholipid structure and the site of action of phospholipases. The phospholipid molecule consists of a glycerol-3-phosphate (blue colour) esterified at its sn-1 and sn-2 positions to non-polar fatty acids (R1 and R2, respectively) and at its phosphoryl group to a polar head group, X. Phospholipase A1 and phospholipase A2 cleave the acyl ester bonds at sn-1 and sn-2, respectively. Phospholipase C cleaves the glycerophosphate bond whereas phospholipase D removes the head group, X. PLA, phospholipase A; PLC, phospholipase C; PLD, phospholipase D. http://lipidlibrary.aocs.org/
OXIDATION OF FATTY ACIDS
In peanuts, caster beans, soybean and Cocos nucifera, the pathway of Alfa oxidative reactions have been established. This mechanism has been found to be very active during the germination of seeds or fruits for they store fatty acid components in large quantities. These reactions occur in cytosol and not in mitochondria.
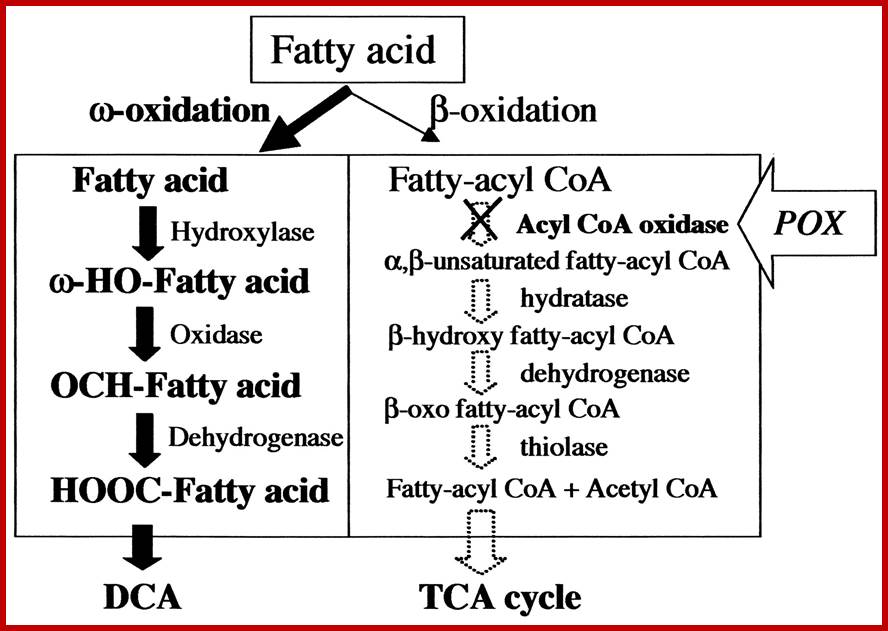
Alternative oxidation pathways for fatty acids in C. tropicalis. Oxidation of fatty acids can occur through β-oxidation (reactions in right panel) or by ω-oxidation of the terminal methyl group to yield the dicarboxylic acid (DCA, reactions in the left panel). Dicarboxylic acids are themselves subject to subsequent β-oxidation. In ω-oxidation, the hydroxylase reaction is catalyzed by members of the CYP52A family of cytochrome P450s and their companion cytochrome P450 reductase. The oxidase reaction is catalyzed by a fatty alcohol oxidase, and the dehydrogenase reaction is catalyzed by a fatty aldehyde dehydrogenase. Inactivation of the POX genes of β-oxidation, which encode acyl coenzyme A (CoA) oxidases, blocks β-oxidation. Under these circumstances, fatty acids are oxidized solely by ω-oxidation, and long-chain dicarboxylic acid homologues of the parent fatty acids accumulate.www.aem.asm.org
In the initial reaction involves the removal of CO2 from the terminal carbonyl group of the fatty acid and the alfa carbon is rendered as an aldehyde group with the help of hydrogen peroxide. This initial step is catalyzed by fatty acid peroxidase enzyme and it requires flavin oxidases as catalysts.
The aldehyde group of the fatty acid is then oxidized to yield corresponding carboxylic acid. The coenzyme involved in this reaction is NAD, where it gets reduced to HADH2.
The above mentioned two step enzymatic degradation is repeated several times to oxidize fatty acids. The alfa oxidative enzymes utilize fatty acids containing 13 to 18 carbon chains. In this process the oxidation is not complete. Still, during stepwise oxidative processes, the energy is trapped in NADH+H molecules. In many cases, the fatty aldehydes produced in these reactions are converted to alcohols and such alcohols are used in the synthesis of waxes.
β-OXIDATION
The process of β-oxidation involves the cleavage of at β-carbon position of the fatty acid chain yielding 2’ carbon keto acyl Co.A and this process occurs within mitochondria. This is in contrast to alfa oxidation which takes place in cytosol. This process is also multistep reactions and the enzymes required are found within the mitochondrial matrix.
ENTRY OF FATTY ACIDS INTO MITOCHONDRIA
Fatty acids are first activated by ATP medicated extra mitochondrial coenzyme A to produce activated fatty acyl Co.A. Then the acyl group of fatty acyl Co. A is transferred to a carrier protein called carnitine found in the mitochondrial membranes. The carrier protein facilitates the entry of fatty acyl across the membranes. As the fatty acyl carnitine complex enters the membrane, at the inner surface the acyl group from the carnitine protein is transferred to the intra mitochondrial Co. A-SH. The enzymes involved are acyl Co. A synthetase and carnitine acyl transferase. This type of facilitated transport of fatty acids into mitochondria is called ‘Fatty acid shuttle’.
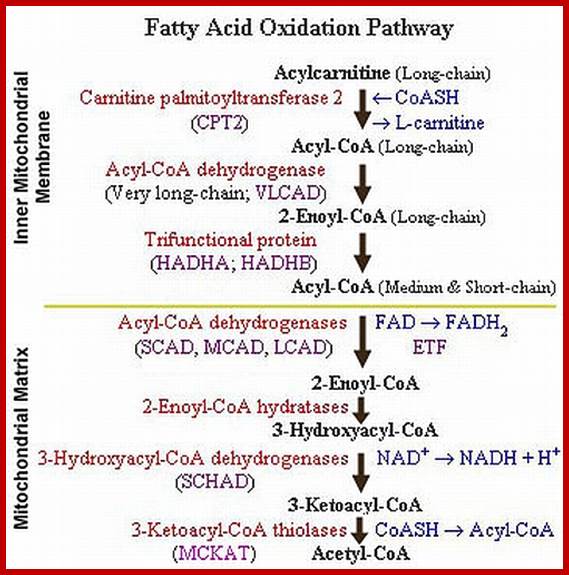
Sometimes the free fatty acids found in mitochondrial matrix are activated rather by GTP THAN ATP. Here palmitic acid has been taken as an example.
FIRST DEHYDROGENATION STEP
The fatty acyl Co. A is first subjected to dehydrogenation reaction by acyl-Co.A dehydrogenase enzyme, where hydrogens are removed from Alfa and B position soft the carbon chain. The coenzyme FAD gets reduced to FADH2 and the product is fatty acyl enoyl. Co.A.
HYDRATION REACTION
This reaction involves the addition of a molecule of water at Alfa & B carbon position to produce 3 hydroxy acyl Co.A. The enzyme involved in this reaction is enoyl Co.A hydratase.
SECOND DEHYDROGENATION STEP
The enzyme-B hydroxy acyl Co.A dehydrogenase catalyses the reaction where both the hydrogens are removed from B carbon position of 3 hydroxyl acyl Co.A to produce 3 keto acyl Co.A. The coenzyme that accepts the hydrogen in this dehydrogenase reaction is NAD.
REMOVAL OF ACETO ACYL CO.A:
He ketoacyl Co.A is then catalyzed by aceto Co.A acetyl transferase where another Co.A – SH is added and acetyl Co.A is removed from the B position. This reaction is also referred to as thiolysis. As these reactions are exergonic they are thermodynamically favored
This process of dehydration, hydration, dehydrogenation and thiolysis as a cycle is repeated again and again, till the entire fatty acid chain is oxidized to 8 acetyl S-Co.A. So the oxidation of one palmitic acid yields 8 acetyl S-Co.A, 7 NADH+ and 7 FADH2.
The acetyl Co.A s produced in this beta oxidation of palmitic acid are drawn into Kreb’s cycle, in which one mole of acetyl Co.A yields 3 NADH+H. one FADH2. They are drawn into inner mitochondrial membrane and the same are subjected to terminal oxidation. As a result, one NADH+H yields 3 ATP and one FADH2 yields 2 ATPs. The overall yield of total energy and ATP by B oxidation of one mole of palmitic acid is as follows.
Simple calculations of the above equations show that 1 mole of palmitic acid on complete B oxidation yields a total energy of 2714 K. Cals, out of which 949 K.cals of energy (excluding 1 ATP used) is stored in 130 ATPs (130 x 7.2 K.cals) produced as the net gain. Thus, the efficiency of B oxidation in terms of net gain of utilizable form of energy is about 35%.
OXIDATION OF UNSATURATED FATTY ACIDS
Unsaturated fatty acids contain one or more double bonds in cis configuration. The usual B oxidation utilizes unsaturated fatty acids and cleaves of acetyl Co.A in the usual way till the oxidising carbon chain is shortened up to unsaturated bonds.
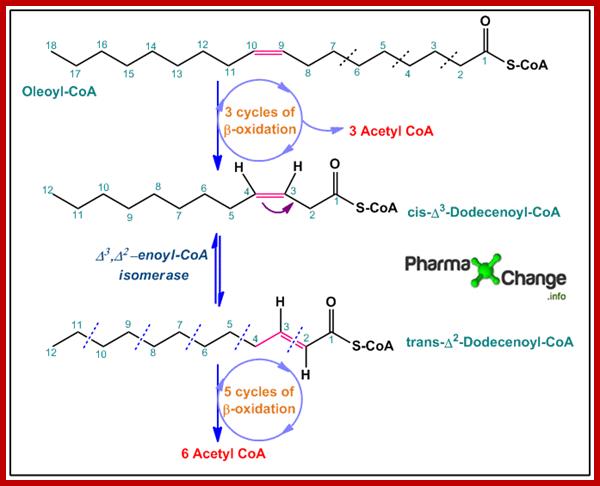
· Oleoyl CoA undergoes three cycles of β-oxidation like normal saturated fatty acids to yield 3 molecules of acetyl CoA and results in the formation of 12-carbon fatty acyl-CoA with a cis double bond now between carbon 3 and 4. This product is known as cis-Δ3-Dodecenoyl-CoA.
· The above product formed has a cis double bond and cannot further participate in β-oxidation. Thus by the action of Δ3,Δ2-enoyl-CoA isomerase, cis-Δ3-Dodecenoyl-CoA is converted to trans-Δ2-Dodecenoyl-CoA. This is the significance of the isomerase enzyme in the β-oxidation of unsaturated fatty acids.
· trans-Δ2-Dodecenoyl-CoA now is acted upon by the enzymes of β-oxidation pathway in five continuous cycles to yield another 6 molecules of acetyl CoA.
The acetyl-CoA molecules now enter the Kreb’s cycle. http://pharmaxchange.info/ BY
SWEETY MEHTA
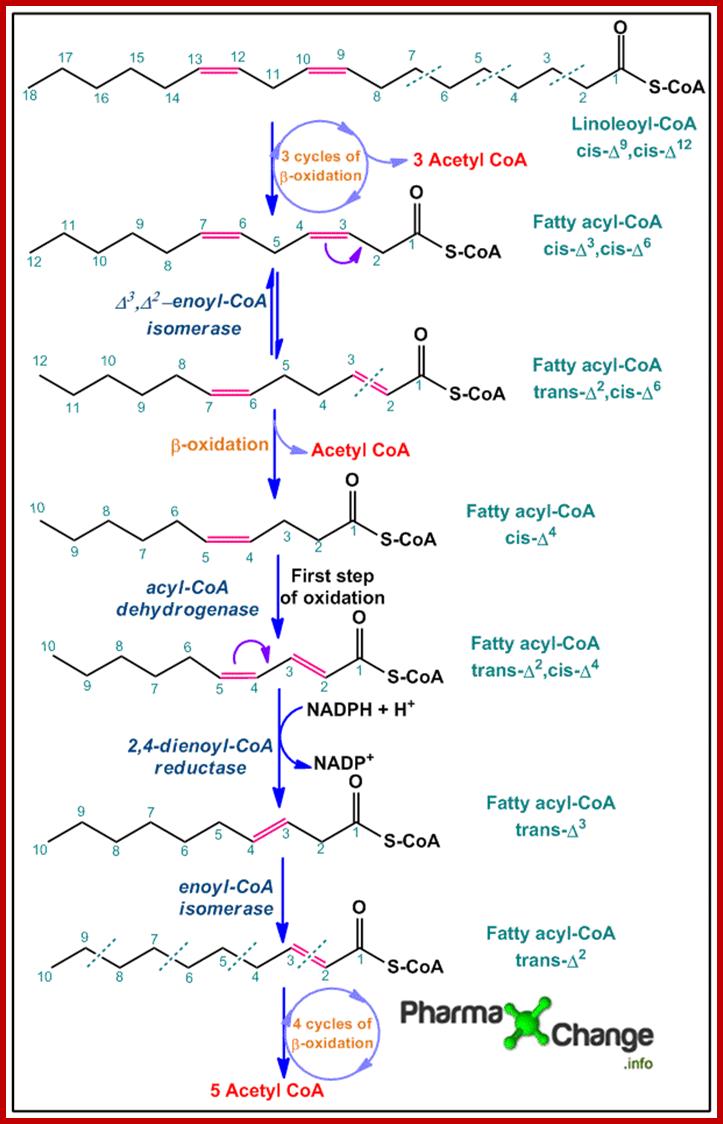
· Linolenic acid is an unsaturated fatty acid with two cis double bonds. Like saturated fatty acids the polysaturated fatty acid undergoes three cycles of β-oxidation to yield three molecules of acetyl CoAalong with a 12 carbon chain fatty acyl-CoA with cis double bonds at position 3 and 6 (cis-Δ3,cis-Δ6).
· Since the mitochondrial enzymes cannot break down cis double bonds, Δ3,Δ2-enoyl-CoA isomeraseconverts it to (trans-Δ2,cis-Δ6) fatty acyl-CoA. The latter product now undergoes one more cycle of β-oxidation to yield the fourth molecule of acetyl CoA and the remaining product left behind is cis-Δ4fatty acyl-CoA.
· By the action of acyl-CoA dehydrogenase, the first step of β-oxidation is achieved, resulting in formation of a double bond at position 2 forming the product (trans-Δ2,cis-Δ4) fatty acyl-CoA. The newly formed product is now acted upon by the enzyme 2,4-dienoyl CoA-reductase to form trans-Δ3 fatty acyl-CoAwhich on further action by enoyl-CoA isomerase gives trans-Δ2 fatty acyl-CoA.
· trans-Δ2 fatty acyl CoA now undergoes four cycles of β-oxidation to yield another five molecules of acetyl CoA.
The acetyl-CoA molecules now enter the Kreb’s cycle.; http://pharmaxchange.info/; BY
SWEETY MEHTA
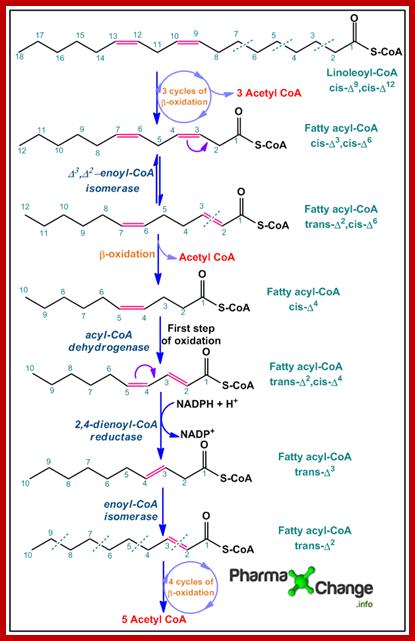
Oxidation of Polyunsaturated fatty acids; http://pharmaxchange.info/ BY SWEETY MEHTA