CARBOHYDRATE METABOLISM
Carbohydrates are defined as organic compounds consisting of polyhydroxy aldehydes or ketones or their derivatives. Carbohydrate means polymers of sugars plus water. The general formula for carbohydrates is (CH2O) n. The most abundant organic compounds available in nature are carbohydrates and they are mostly produced by plants. Nearly 450 billion tons of carbon along with 13 x 1018 K. cals of solar energy is fixed into carbohydrates annually by land plants. Aquatic Plants (including marine) plants also fix substantial amount larger than land plants of dissolved carbon. Carbohydrates thus act as the primary food and fuel for all living organisms and also it is the main source of energy for various human needs like firewood, coal, biogas, petroleum, and bio-electricity etc. It also provides Carbon skeletal components for the other organic compounds.
|
||||||
|
||||||
|
||||||
|
http://www2.chemistry.msu.edu/
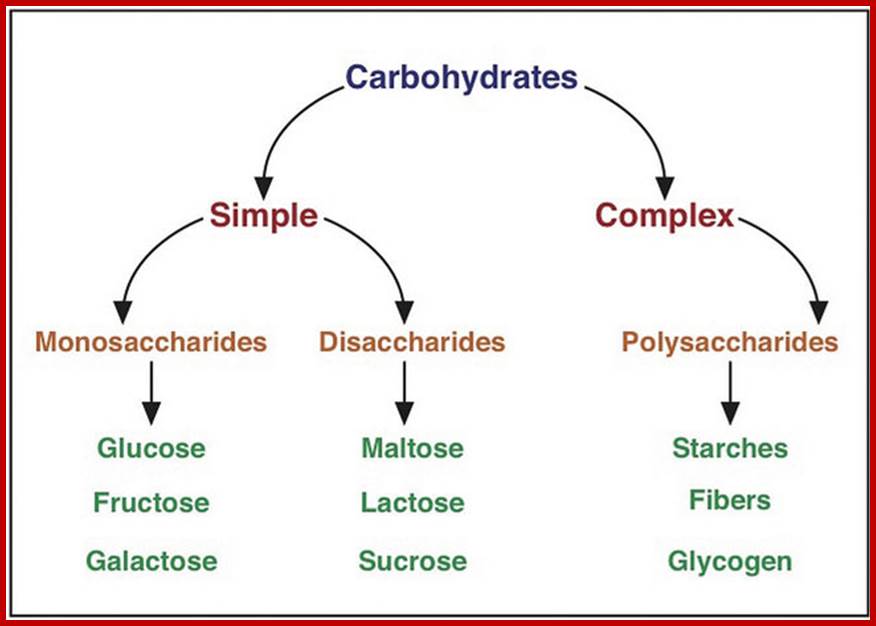
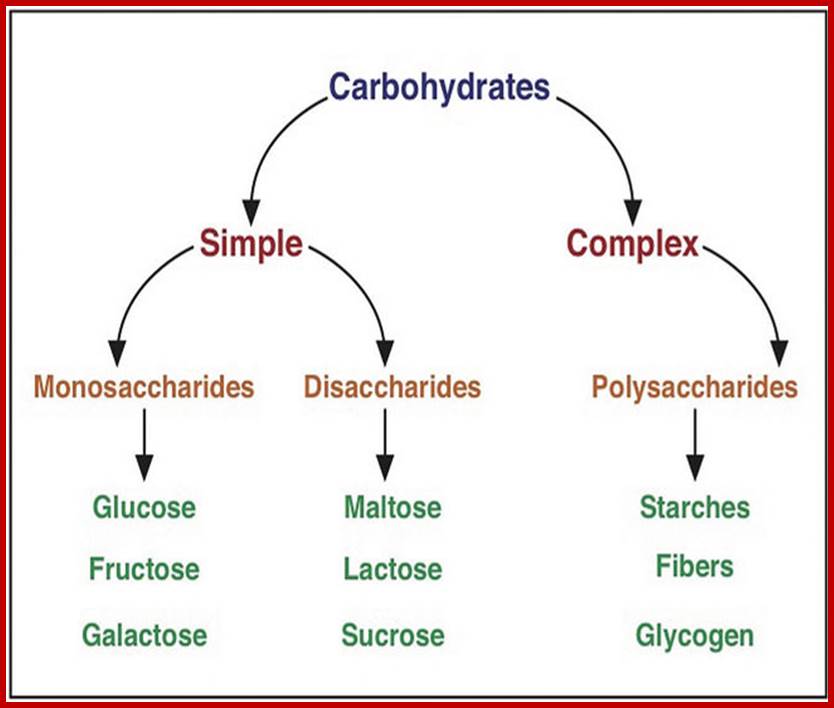
BASIC UNITS OF CARBOHYDRATES
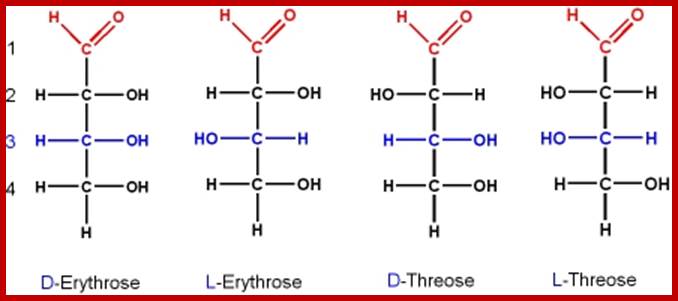
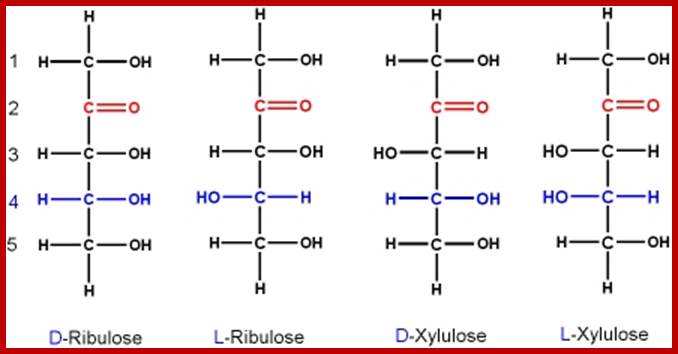
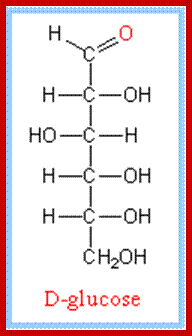
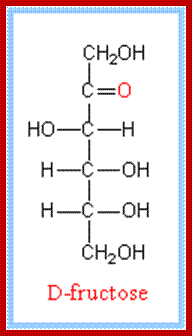
Basic units of carbohydrates are monosaccharides with an empirical formula of (CH2O)n where n=3 or more. Depending upon the number of carbon atoms present in a single molecule, they are named as Triose (3C), Tetrose (4C), Pentose (5C), Hexose (6C), Heptose (7C), etc. In the above said molecules, except one carbon atom all other carbons possess hydroxyl groups. If the carbonyl group (CHO) is present at the terminal region, such sugars are called Aldoses; on the contrary, if C=O group is present in any other position, they are called ketoses.

www.time-to-run.com
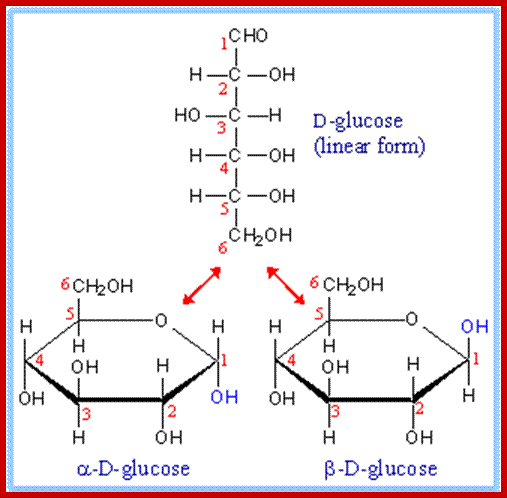
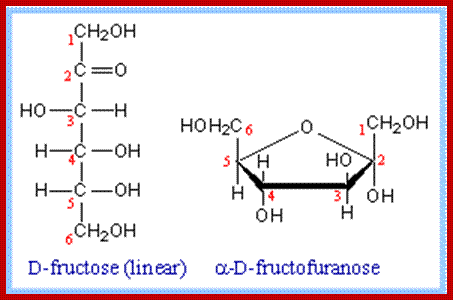
Almost all monosaccharaides exist as Aldoses as well as ketoses and each of them exhibit optical isomerism (Dextro & Levo forms). Paradoxically, most of the monosaccharaides that are biologically active are D forms. Furthermore, each of the D forms may show a or B structures. Now it is known that D glucose and D-Fructoses exist not as open chain structures but as six members closed ring structures i.e. glucose as a – D glucose pyranose and fructose as a -D fructose furanose. Even pentoses exist as ring like structures. Ex. Ribose, Deoxyribose, etc.


Lewis Structures; http://study.com/academy
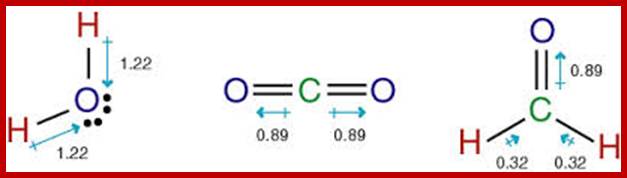
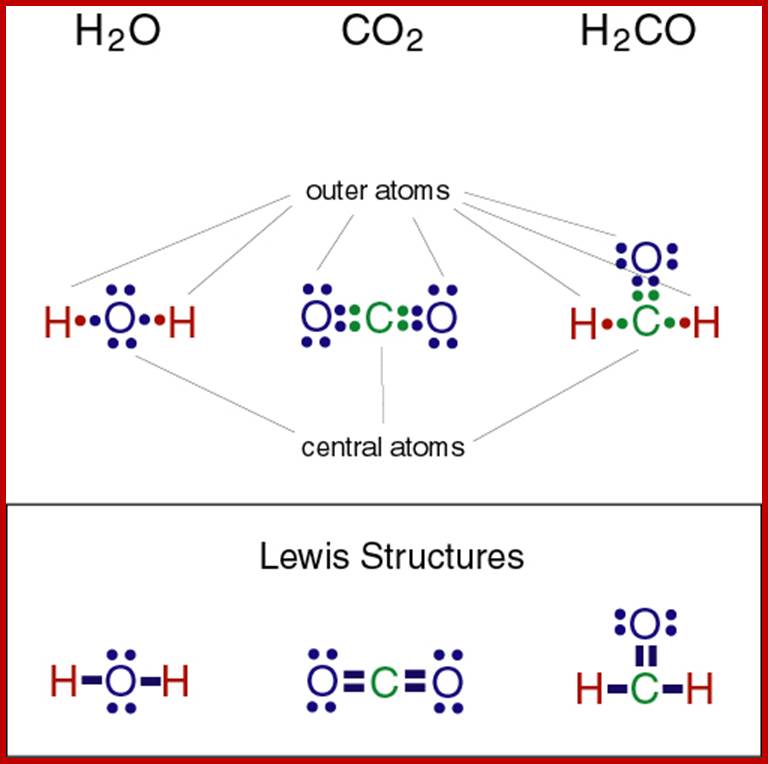
H2O, CO2 and H2CO; http://www.marin.edu/
Lewis structures

Resonance- http://chemwiki.ucdavis.edu/
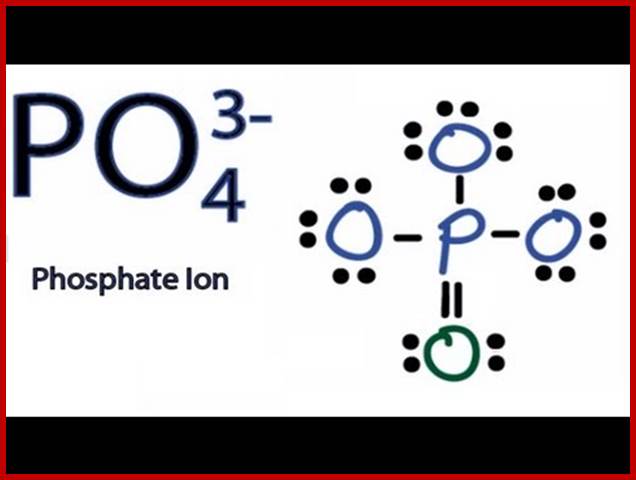
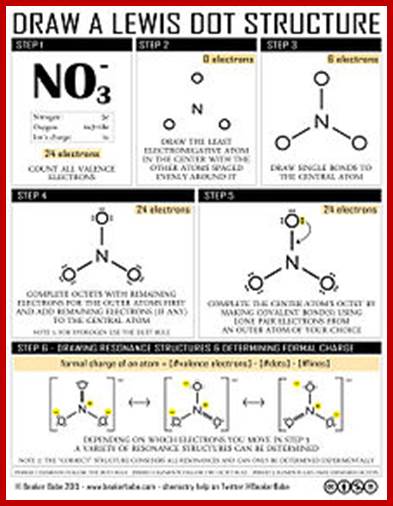
A simple representation of how Lewis structure one can draw;www.wekipedia.org
Gilbert N. Lewis diagrams show bonding between atoms of a molecule and a lone pair of electrons if exist in a molecule A Lewis structure can be drawn for any covalently linked molecules and also to any compound with coordinate compounds.
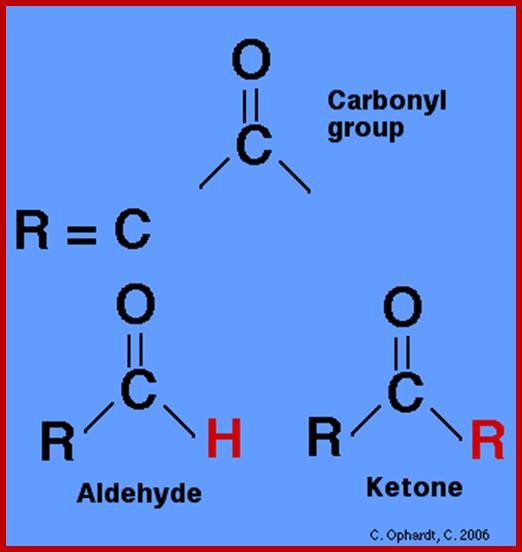

Trioses- Aldehyde and Ketose; www.users.humboldt.edu;
http://www.mikeblaber.org/
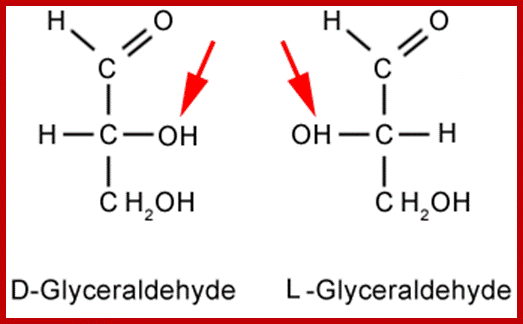
Isomers: www.ncl.ac.uk
Aldose monomers; http://www.mikeblaber.org/
Ketose monomers; http://www.mikeblaber.org/
Among the hexoses, glucoses act as building blocks for starch and cellulose, which form the bulk of the plant products. Among the pentoses, ribose and deoxyribose are the most important components of nucleic acid. Many monosaccharides also exist as glycosidic O-acyl or C-methyl derivatives and some may exist as alcohols, acids, phosphates or amino sugars.

"Family
tree" of the D -aldoses. By appending CH-OH groups to extend the basic
framework, so more K-sugar can be derived (from trio with three C-hexoses to
six carbon atoms):. Here, the
rotation of polarized light with (+) and) (specified -. ( 1 ) D -(+)-
glyceraldehyde , ( 2 ) D -(-)- erythrose , ( 2b ) D -(-)- threose , ( 3a ) D
-(-)- ribose , ( 3b ) D - ( -) - arabinose , ( 3c ) D -(+)- xylose , ( 3d ) D
-(-)- xylose , ( 4a ) D -(+)- aloe , ( 4b ) D -(+)- altrose ; ( 4c ) D -(+)-
glucose , ( 4d ) D -(+)- mannose , ( 4e ) D -(-)- glucose , ( 4f ) DK -(-)-
idose ; ( 4g ) D - (+ ) - galactose , ( 4 h ) D -(+)- talose; Basic
monosaccharaides; Aldoses; www.polysac3db.cermav.cnrs.fr;
https://en.wikibooks.org/wiki/
Howorth projection; https://en.wikibooks.org
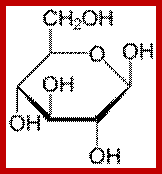
Alpha D Glucopyranose; Beta-D-glucopyranose; https://en.wikibooks.org/wiki/
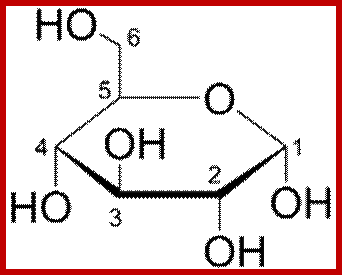
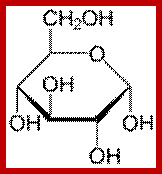
The three-dimensional structure of a monosaccharides in cyclic form is usually represented by its Haworth projection. In this diagram, the α-isomer has the OH- of the anomeric carbon below the plane of the carbon atoms, and the β-isomer has the OH- of the anomeric carbon above the plane. Pyranoses typically adopt a chair conformation, similar to cyclohexane. In this conformation the α-isomer has the OH- of the anomeric carbon in an axial position, whereas the β-isomer has the OH- of the anomeric carbon in equatorial position.
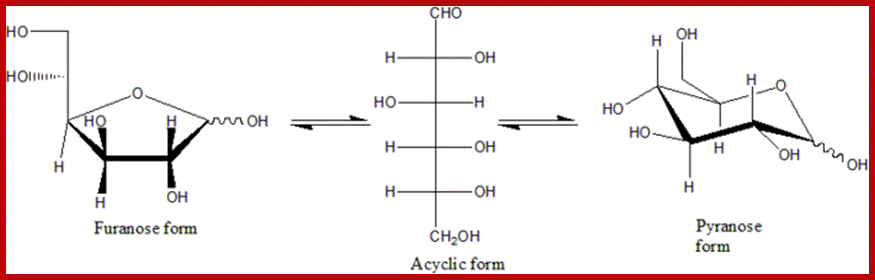
Furanose, Acyclic and Pyranose forms; https://en.wikibooks.org/wiki/
https://www.rpi.edu
http://biology.tutorvista.com/
DI and POLYSACCHARIDES
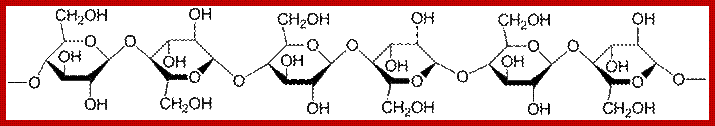
Two monosaccharides, if linked by a glycosidic bond such a compound is called a disaccharide. If three are linked, then the compound is called a trisaccharide. The most common disaccharides found in plants are maltose, cellobioses sucrose and gentiobose. Among trisaccharide, raffinose and (melezitose?) are found in sugar beets, root tubers and coniferous trees.
Maltose; http://chemistry2.csudh.edu/http://www.rpi.edu/

Disaccharides; www2.estrellamountain.edu
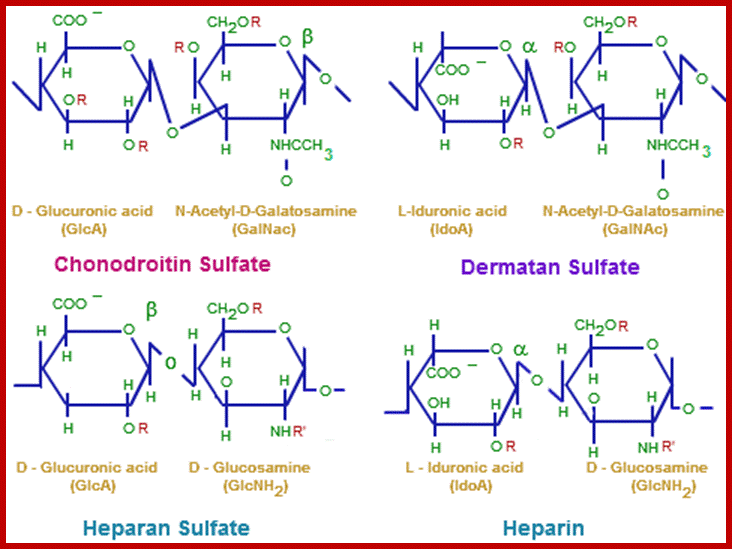
Glycosamine- glycans;chemistry.tutorvista.com
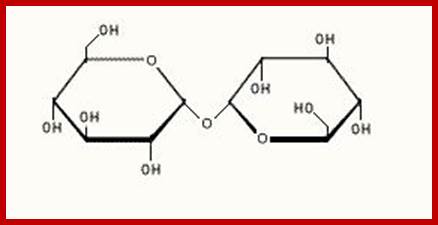
Trehalose; http://www.hdac.org/
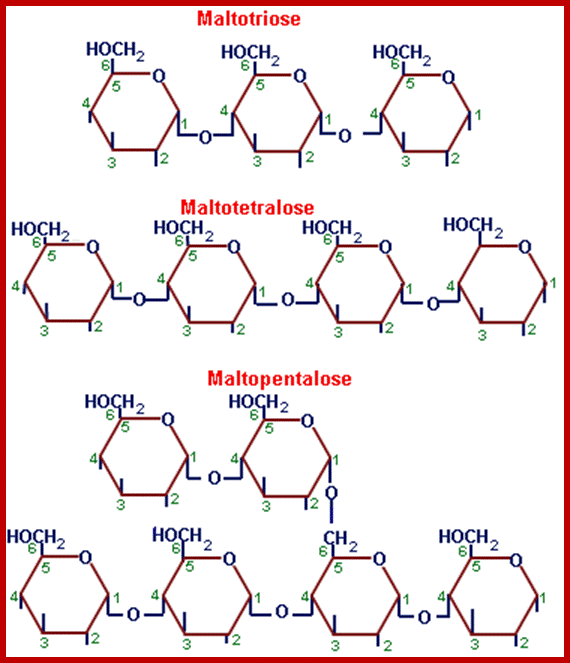
http://chemistry.tutorvista.com/
POLYSACCHARIDES
Polysaccharides are those carbohydrates that are made up of three or more monosaccharides linked by glycosidic bonds. Plants contain a variety of polysaccharides of which starch and cellulose are the major carbohydrate components of the plant body.
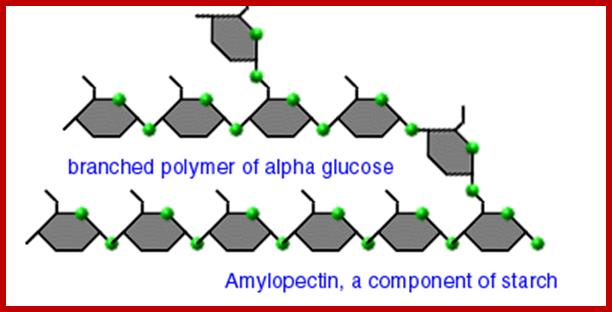
http://www.brooklyn.cuny.edu/
Oligosachharides;
Raffinose and Raffinose family:
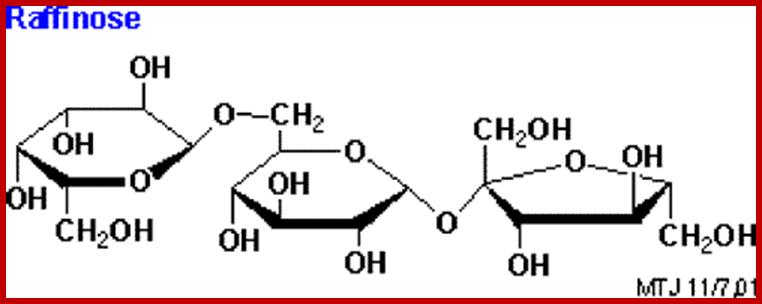
Raffinose; ibiochembuzz.wordpress.com; Photo credit – http://www.biotech.iastate.edu/lab_protocols
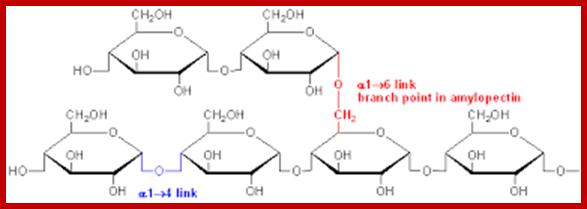
Alpha 1-6linkage; http://chemistry.tutorvista.com/
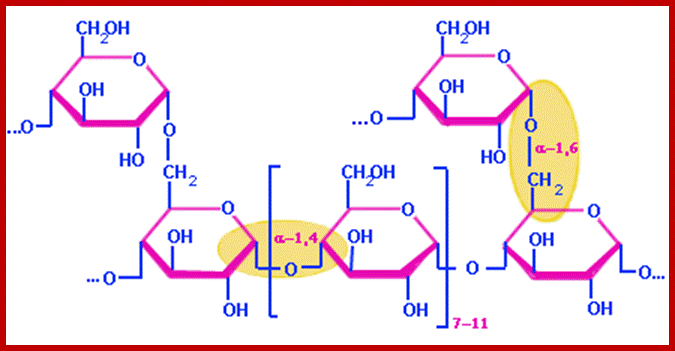
Just like amylopectin, dextran a polysaccharide, also contains alpha 1-6 glycosidic linkage in main chain and side branches are bonded with alpha 1-3 and alpha 1-4 glycosidic linkage. http://chemistry.tutorvista.com/
Starch:
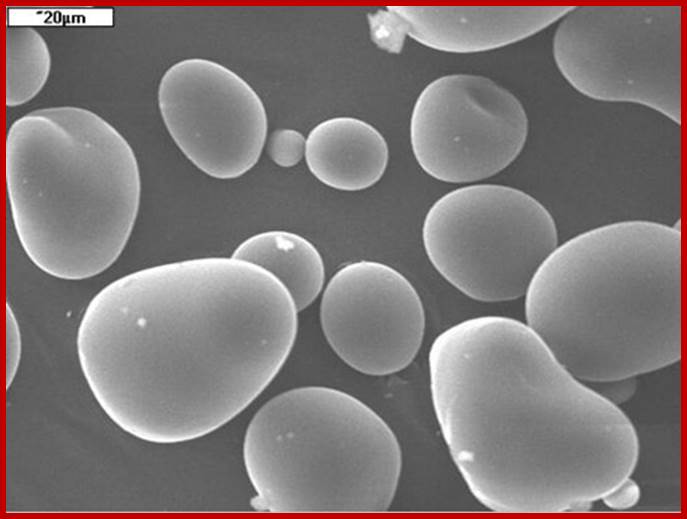
Native potato starch granules; http://www.ejpau.media.pl/
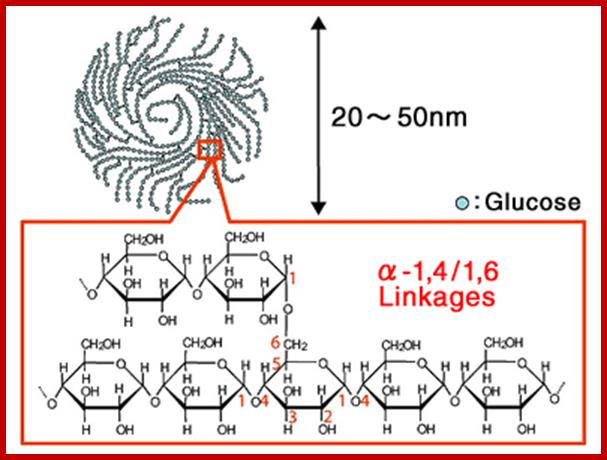
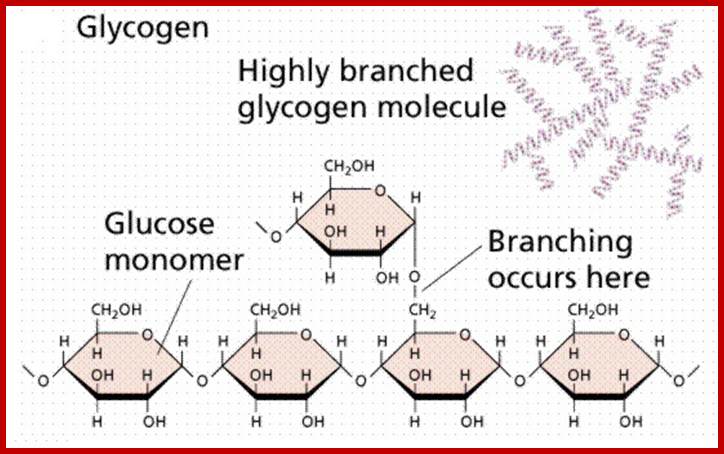
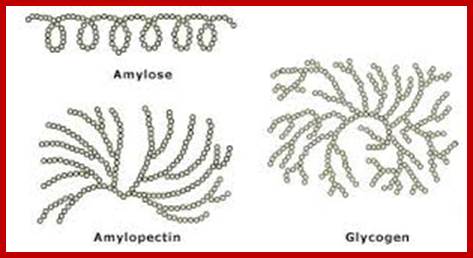
Glycogen- polysaccharide of glucoses; amylopectin- www.glico.co.jp
1-6 branching; david-bender.co.uk
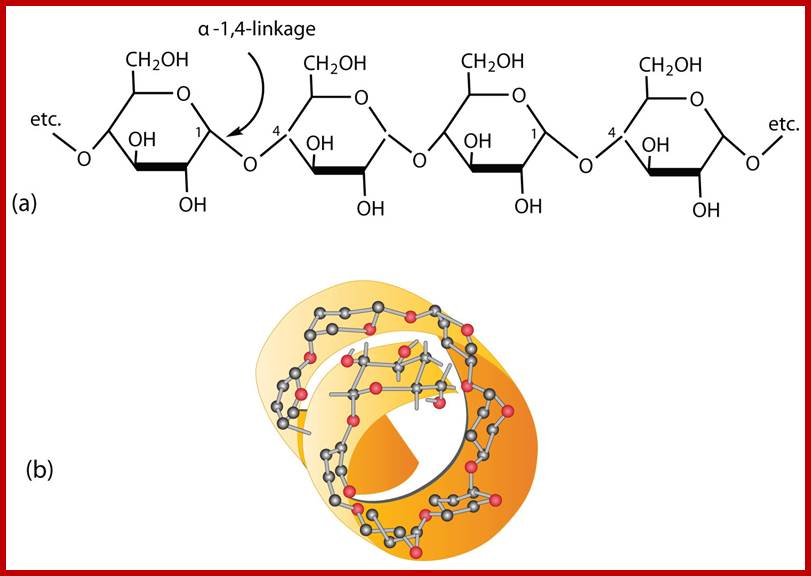
(a) Amylose is a linear chain of α-D-glucose units joined together by α-1,4-glycosidic bonds. (b) Because of hydrogen bonding, amylose acquires a spiral structure that contains six glucose units per turn. http://2012books.lardbucket.org/
Glycogen-made out of glucoses with 1-6 bonding to generate branching; winnieboo.blogspot.com
http://medicaltextboks.blogspot.in/
Starch granules; www.visualphotos.com
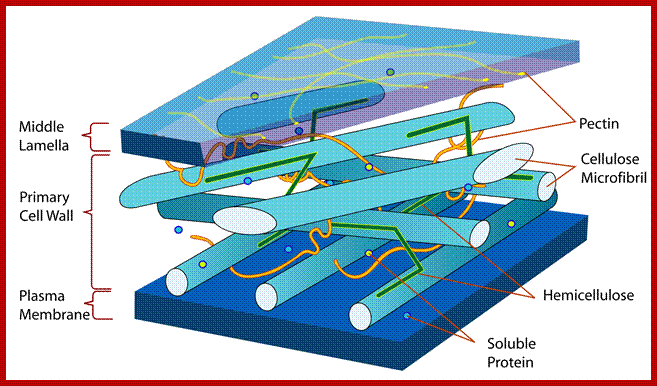
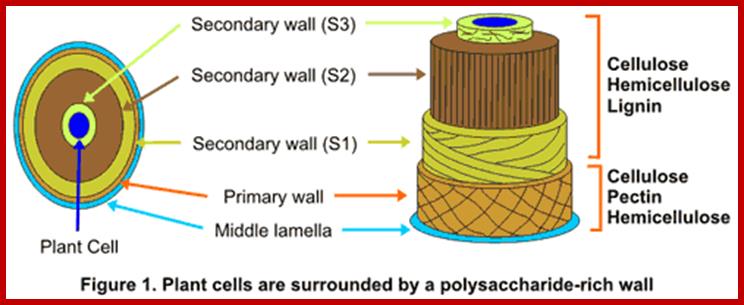
PLANT CELL WALL COMPONENTS
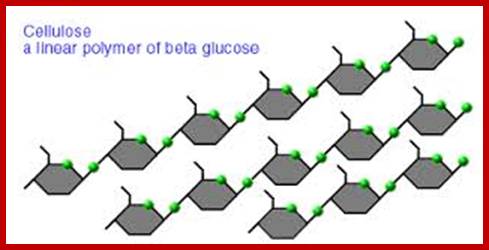
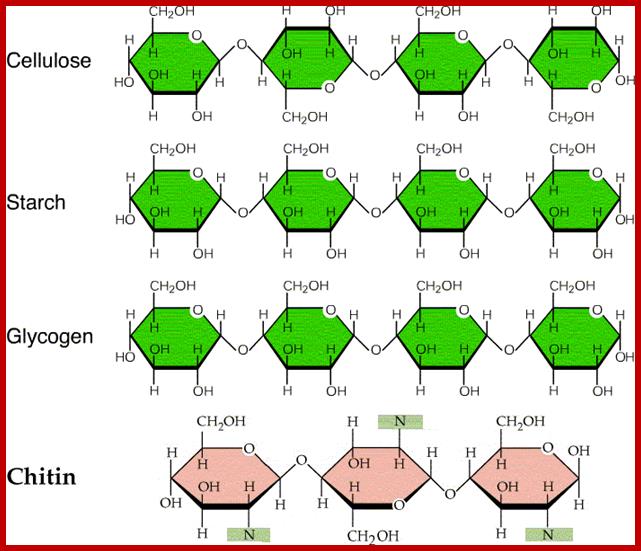
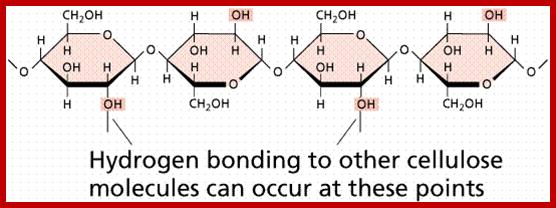
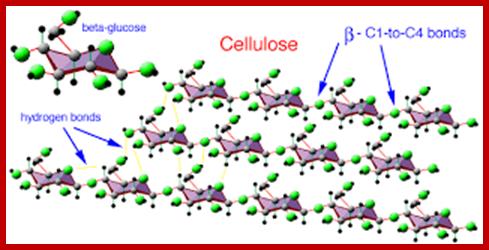
Cell wall is mainly made up of cellulose, hemicellulose, pectin and extensin, the latter is a polypeptide chain. Cellulose acts as the main fibrous component of the cell wall, but pectin and hemicellulose act as matrix components in which cellulose fibrils are embedded. Cellulose is a polymer of β-D glucose units held by (1-4) glycosidic bonds. Such chains may be made up of 1000-1500 glucose residues, whose mol. wt my range from 150,000 to 250,000 Daltons. Many such cellulose chains aggregate into bundles called micelles and micelles into micro fibrils. Micelles are interconnected with few cellulose fibers. Cellulose, though hydrophilic in nature, it is insoluble in water. Almost pure cellulose can be found in cotton fibers.
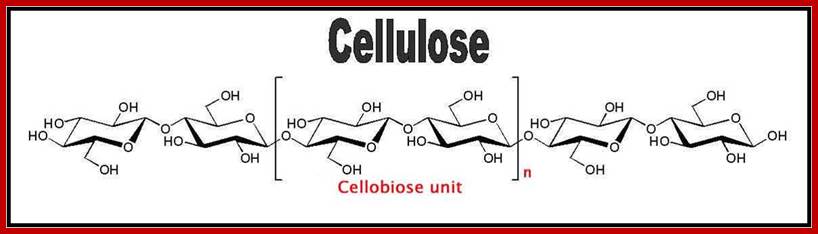
www.lycaeum.org; www.paperpools.blogspot.com
www.en.wikipedia.org; http://peakenergy.blogspot.in/
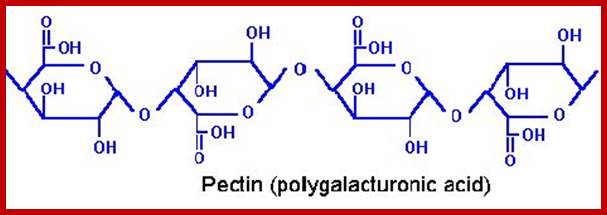
Pectin, like our common cment, is the major binding component of the cell walls of plants and fruits, It is chemically a polysaccharide, consisting of a linear chain of linked molecules of galacturonic acid; regions on the backbone with a lot of side chains are the so-called “hairy regions”, and regions with little side chains are referred to as “smooth regions”.www.food-info.net.
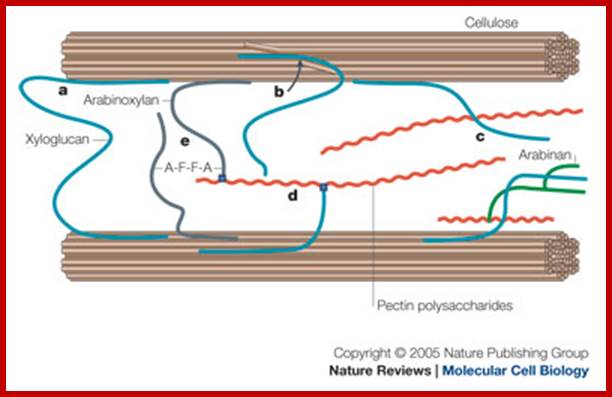
Growth of the plant cell wall; The distinctive pectin
domains are believed to be covalently crosslinked to each other (see figure,
part a), but the nature of the crosslink has not been
determined. In addition, two other types of linkage involving boron (part b) and calcium (part c) are
important crosslinking mechanisms. Part a of the figure shows a model of how the
pectin domains may be covalently linked together to form a massively large
macromolecular pectin network. This is a simplified version of a recent model
by Vincken et al.4,
in which rhamnogalacturonan I serves as the backbone and the other pectin
domains are attached as branches. Homogalacturonans are ionically crosslinked
by calcium (part c)
whereas boron crosslinked rhamnogalacturonan II through diester linkages (part b).
Rhamnogalacturonan II forms dimers through a borate ester bond (part b). This
crosslinking is important for normal wall formation as well as for the control
of wall porosity and wall thickness. Homogalacturonan (also known as
polygalacturonic acid) forms stiff gels through Ca2+-mediated
crosslinking of its carboxyl groups through ionic and COORDINATE BONDS (part c). Growing cells
usually synthesize homogalacturonan in which 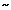 75% of the carboxyl groups (COO-) are methyl esterified
(COOMe) — this modification removes the negative charge of the carboxylate ion
and blocks its ability to undergo Ca2+crosslinking. Highly
esterified homogalacturonans do not form stiff gels and their secretion might
help the expanding wall to remain pliant. Carboxyl-based crosslinking sites are
unmasked later, as the cells cease growth, owing to the action of pectin
methylesterases. These methylesterases, which are secreted by plant cells into
their wall space, hydrolyse the methylesters and free the carboxyl group for Ca2+crosslink
formation and gel formation. http://www.nature.com/
75% of the carboxyl groups (COO-) are methyl esterified
(COOMe) — this modification removes the negative charge of the carboxylate ion
and blocks its ability to undergo Ca2+crosslinking. Highly
esterified homogalacturonans do not form stiff gels and their secretion might
help the expanding wall to remain pliant. Carboxyl-based crosslinking sites are
unmasked later, as the cells cease growth, owing to the action of pectin
methylesterases. These methylesterases, which are secreted by plant cells into
their wall space, hydrolyse the methylesters and free the carboxyl group for Ca2+crosslink
formation and gel formation. http://www.nature.com/
Structure of xyloglucan; principal component of the hemicelluloses. The heptamer block is shown (glucan 4-xylose 3). In blue backbone β-D-glucans; in red α-D-xylose; in black α-D-galactose and in brown α-L-fucose residues. http://www.intechopen.com/
Glycerol treatment leads to breakdown products-Lignin, hemicellulose and cellulose; www.wholesaleinvestor.com; www.purdue.edu
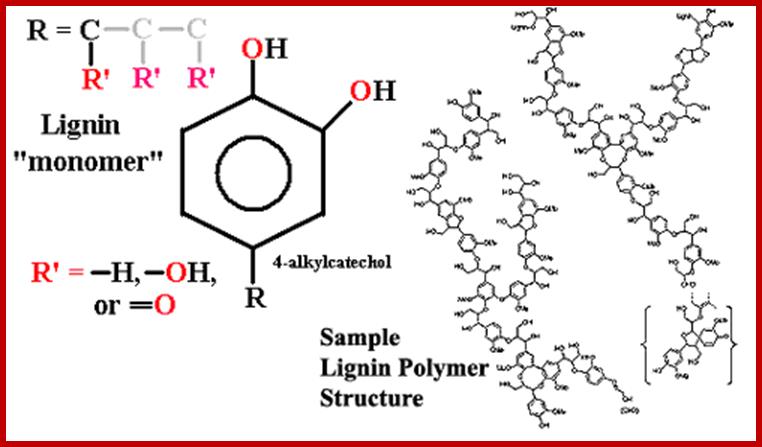
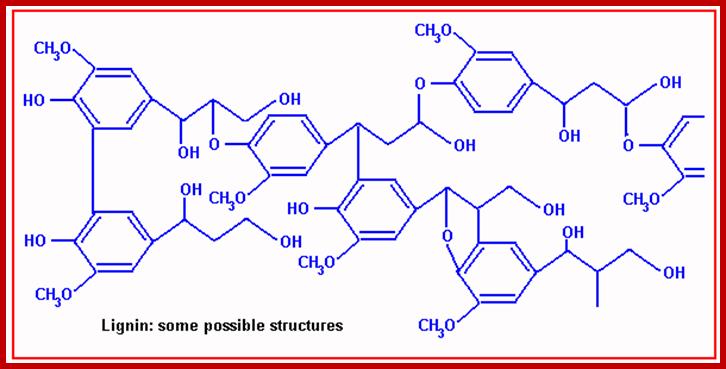
Lignin polymers; www.palaeos.com
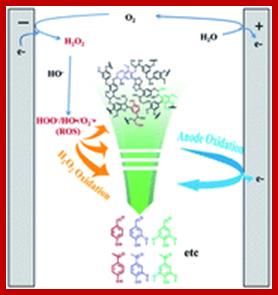
Lignin is a natural aromatic macromolecule in huge quantity and might serve as sustainable resources for the chemical industry after being depolymerized. An electrochemical approach combining anode oxidation and electro-generated H2O2 oxidation has been developed for converting lignin into value-added aromatic chemicals in this study. Lignin in alkali solution was electrolyzed in an undivided electrolytic cell with a cylindrical graphite felt cathode and a RuO2–IrO2/Ti mesh anode, in which the by-product O2 on the anode could be efficiently reduced to H2O2 on the cathode in situ. Results display that the depolymerization productivity via the integrated approach obviously surpassed the sum of that by separate H2O2 oxidation and anode oxidation. Moreover, the analysis results of GC-MS, GPC, and C9 expanded formula confirmed that C–C bonds and C–O–C bonds in lignin were cleaved synergistically by direct anodic oxidation and indirect H2O2 oxidation, and the macromolecules are gradually depolymerized into final products of monomers and dimers. http://pubs.rsc.org/
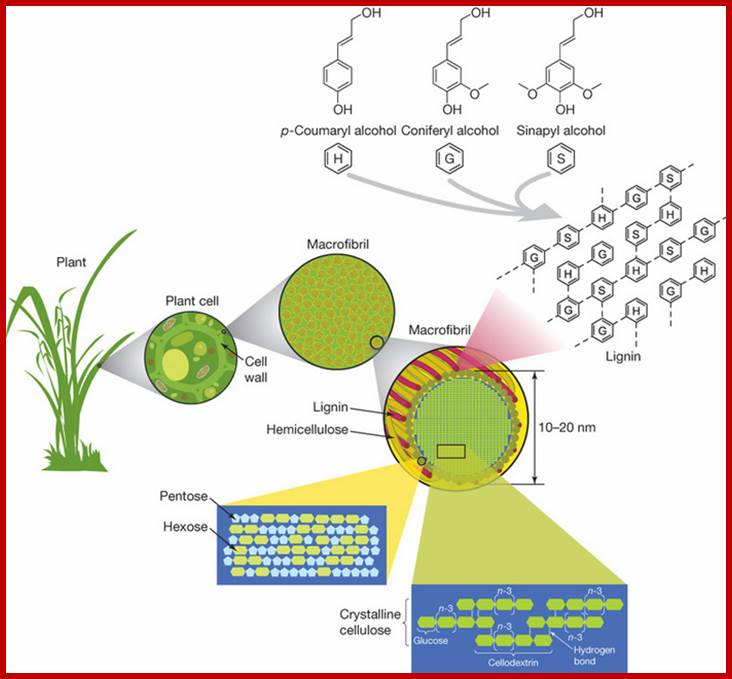
Cellulose beta 1-4 linked chains,5 to 6 carbon sugars-arabinose, Galactose, glucose, mannose and xylulose and lignin-polymer of 3 major phenolic components such as p-coumaryl alcohol (H), coniferyl alcohol (G) and sinapyl alcohol(S) ,;Structure of Lignocellulose; www.nature.com
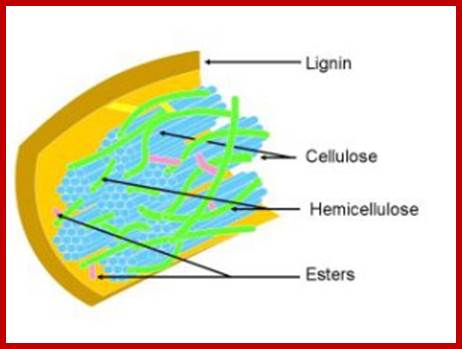
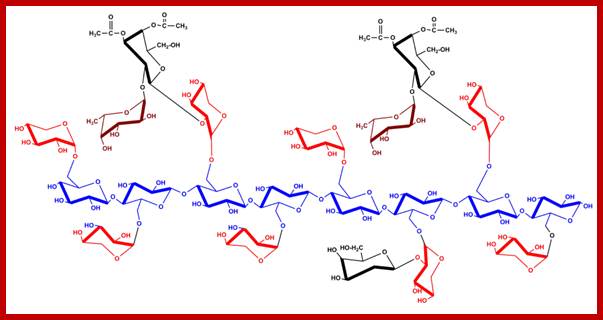
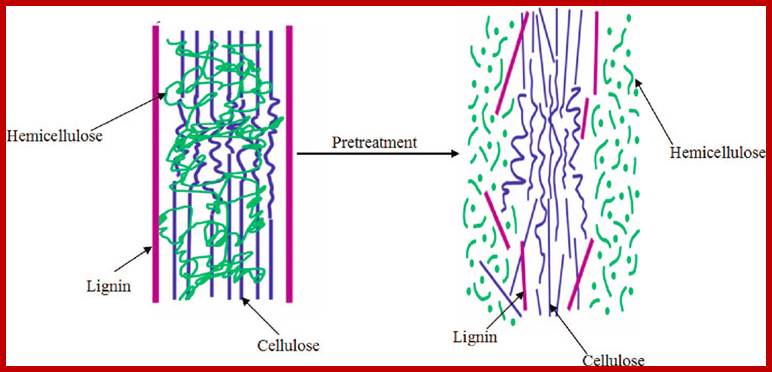
This is a complex structure in which the cellulose is surrounded by a monolayer of hemicellulose and embedded in a matrix of hemicellulose and lignin. Furthermore lignin specifically creates a barrier to enzymatic attack while the highly crystalline structure of cellulose is insoluble in water while the hemicellulose and lignin create a protective sheath around the cellulose. This structure can be seen in the image below. This structure of lignocellulose therefore plays a huge role in inhibiting degradation of the hemicellulose and cellulose structure to monomeric sugars which is necessary to effectively convert biomass into ethanol. Processing of lignocellulose is therefore essential for the conversion of lignocellulosic biomass to biofuel such as bio-ethanol; lignofuel.wordpress.com
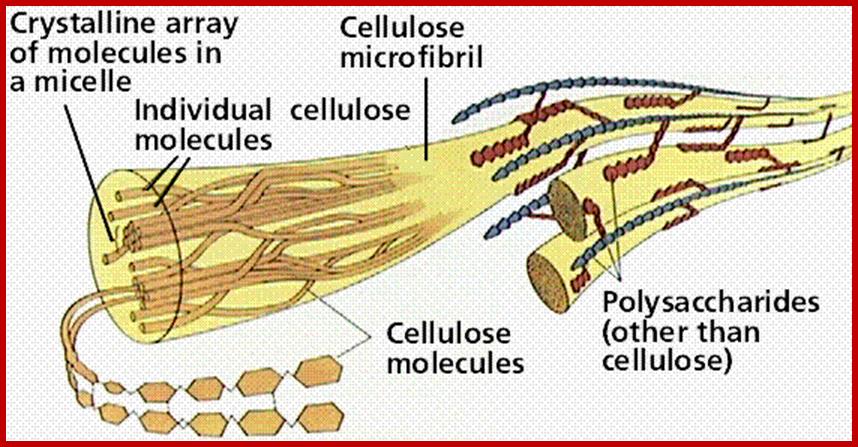
www.sumarsih07.files.wordpress.com
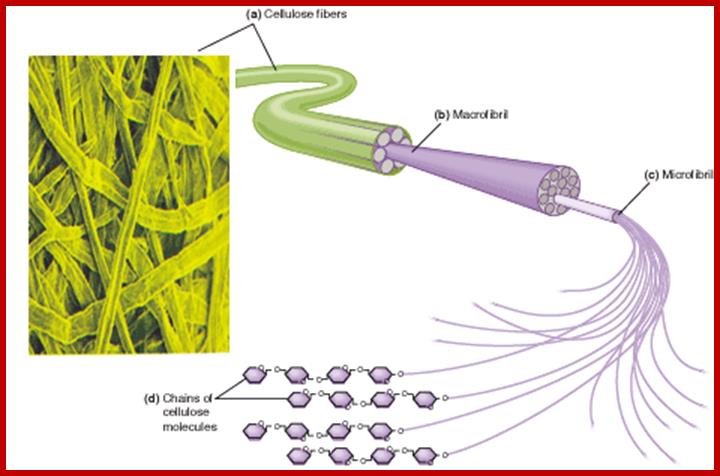
Cellulose structure is composed of a long, linear polymer of 1, 4 J3-linked glucose units that is found in the plant cell wall. Hydrogen bonding between sugar residues in adjacent parallel running cellulose chains imparts a micro fibril three-dimensional structure to cellulose. Being a large, linear, neutrally charged molecule, cellulose is water-insoluble, although it can be modified chemically (e.g., sodium carboxymethyl cellulose) to be more soluble and used as an additive in foods. Degradation of cellulose by colonic bacteria varies, but generally it is poorly fermented (doesn’t digest well). Some examples of foods high in cellulose relative to other fibers include bran, legumes, peas, root vegetables, vegetables of the cabbage family, outer covering of seeds, and apples.www.desertbruchid.net
Cellulose fibers; 6my.dromagal.top
![]()
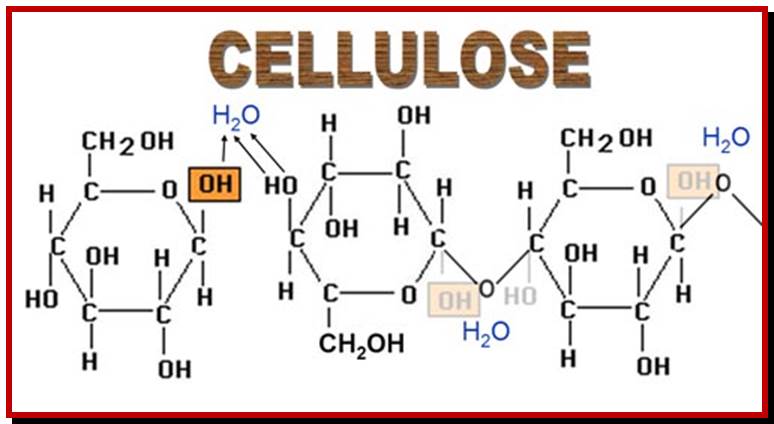
www.chemistryland.com![]()
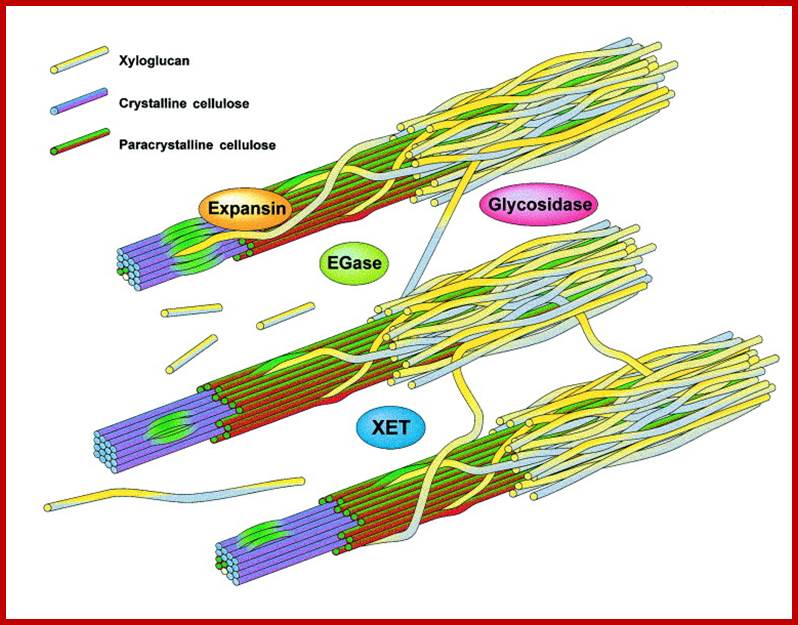
Modification of the plant primary cell wall is required for both cell expansion and for developmental events, such as fruit softening, where cell size remains static but where wall loosening is an important feature. Recent studies suggest that the cellulose–xyloglucan network is targeted by similar enzymatic activities in both expanding cells and ripening fruit but that unique isoforms are expressed in each process. Disassembly of this structural network probably involves the concerted and synergistic action of suites of these enzyme families, where one family of cell wall modifying proteins might mediate the activity of another, providing the basis for orchestrating ordered cell wall restructuring and turnover. www.cell.com

Bamboo fiber, uses high quality natural bamboo as raw material, special tech process bamboo cellulose is extracted, then glue spinning processes and manufacturing regenerated cellulose fiber. Without any chemical additives, has good air permeability, water absorption, strong wear resistance, good dyeability features, known as the traditional four fibers (cotton, linen, wool, silk) five new fibers, breathing eco-textile! Fiber Queen! Bamboo fibers; http://www.aliexpress.com/
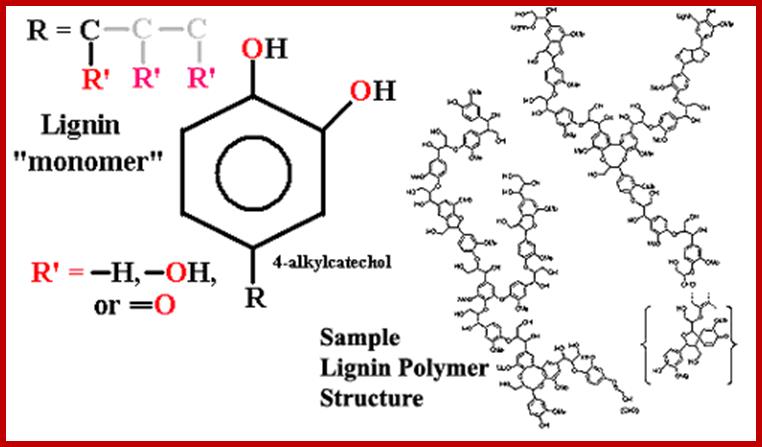
Lignin polymers;www.palaeos.com
Lignin is a natural aromatic macromolecule in huge quantity and might serve as sustainable resources for the chemical industry after being depolymerized. An electrochemical approach combining anode oxidation and electro-generated H2O2 oxidation has been developed for converting lignin into value-added aromatic chemicals in this study. Lignin in alkali solution was electrolyzed in an undivided electrolytic cell with a cylindrical graphite felt cathode and a RuO2–IrO2/Ti mesh anode, in which the by-product O2 on the anode could be efficiently reduced to H2O2 on the cathode in situ. Results display that the depolymerization productivity via the integrated approach obviously surpassed the sum of that by separate H2O2 oxidation and anode oxidation. Moreover, the analysis results of GC-MS, GPC, and C9 expanded formula confirmed that C–C bonds and C–O–C bonds in lignin were cleaved synergistically by direct anodic oxidation and indirect H2O2 oxidation, and the macromolecules are gradually depolymerized into final products of monomers and dimers. http://pubs.rsc.org/
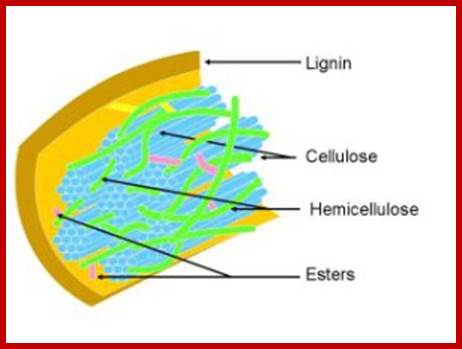
This is a complex structure in which the cellulose is surrounded by a monolayer of hemicellulose and embedded in a matrix of hemicellulose and lignin. Furthermore lignin specifically creates a barrier to enzymatic attack while the highly crystalline structure of cellulose is insoluble in water while the hemicellulose and lignin create a protective sheath around the cellulose. This structure can be seen in the image below. This structure of lignocellulose therefore plays a huge role in inhibiting degradation of the hemicellulose and cellulose structure to monomeric sugars which is necessary to effectively convert biomass into ethanol. Processing of lignocellulose is therefore essential for the conversion of lingo-cellulosic biomass to biofuel such as bio-ethanol; lignofuel.wordpress.com
Lignin- compound chemistry
http://bioenergy.ccrc.uga.edu/
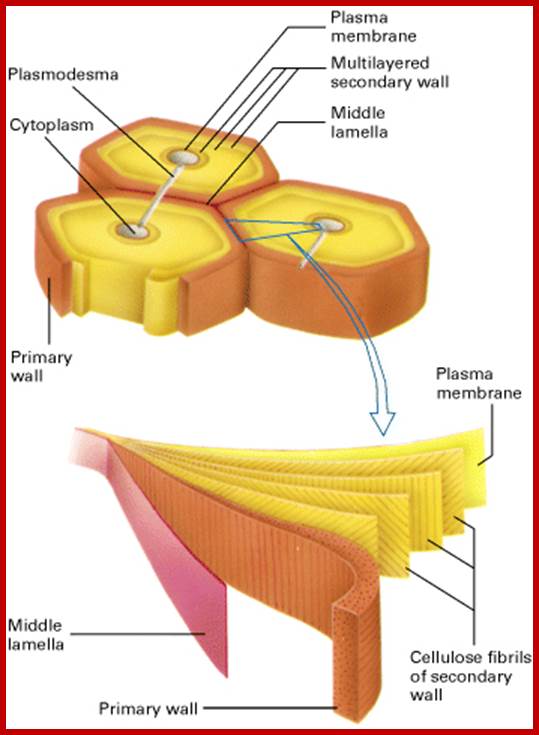
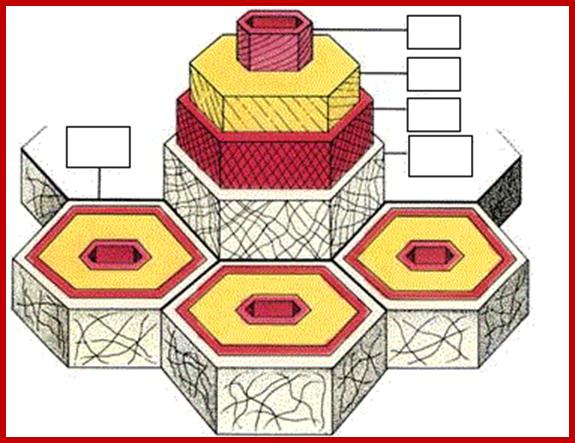
Cell wall layers-Primary to Secondary wall layers
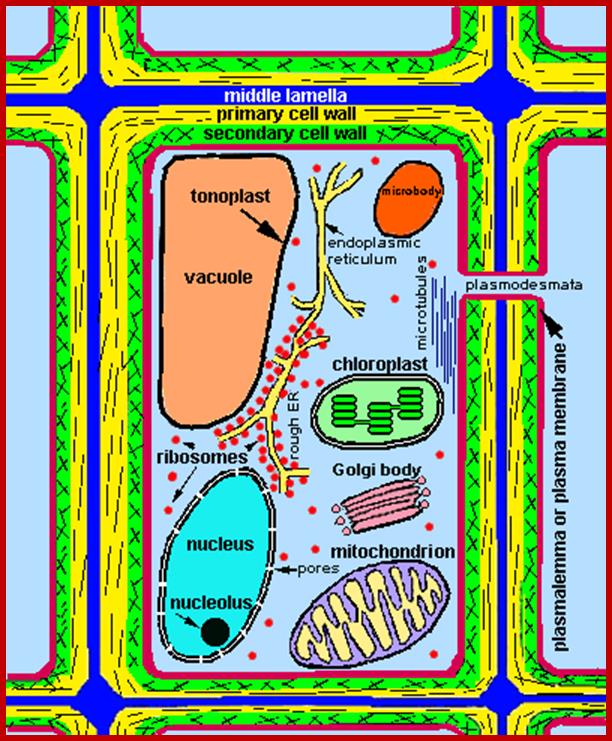
Plasmodesmata; http://www.bio.miami.edu/
A group of cellulose fibrils in the form of micelles are surrounded by an amorphous matrix, which acts as the cementing material. It is mainly made up of polymers of rhamnogalactorunans, arabinogalactans, and polygalacturonans as pectic polymers and xyloglucans with arabinose and galactose as hemicellular materials. On the other hand, extensins is a hydroxyproline rich polypeptide chain, which act as cross link between cellulose and hemicellulose materials. Among cell wall polysaccharides, cellulose is extremely important for the mankind.
Recent studies have revealed that some oligosaccharides of the cell wall have regulatory role on cellular metabolic activities, growth and differentiation.
STARCH
Plants synthesize and store starch as, reserve food material in specialized organs like root tubers, stem tubers, seeds and fruits. Starch granules are mostly deposited in amyloplasts. The important constituents of starch are amyloses and amylopectoses, the latter is always found in excess. The way nature of the pea seeds to be wrinkled and round is due to the presence of excess of starch, but the wrinkled is due to amylopectins.
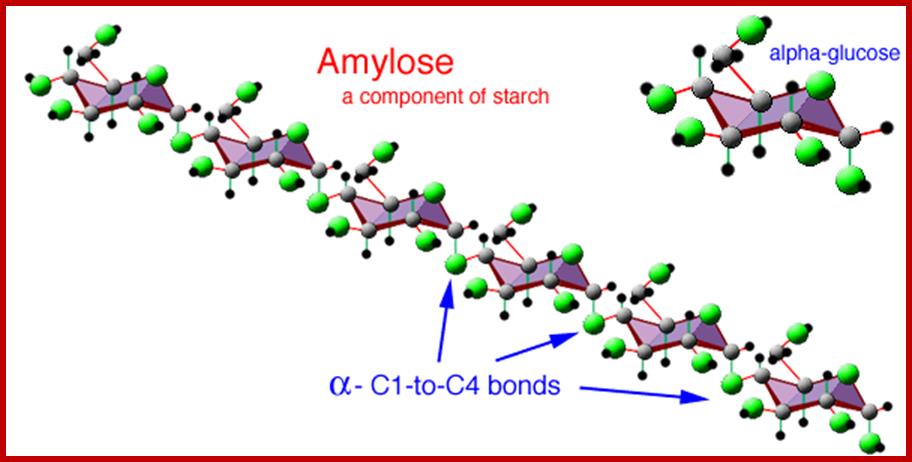
AMYLOSE
Amylose is an unbranched long chain of a -D glucose units held by a1 – 4 glycosidic bonds. These molecules show helical coiling with six glucose units per turn. They are not soluble in water but with water they form hydrated micelles, which give blue color with iodine.
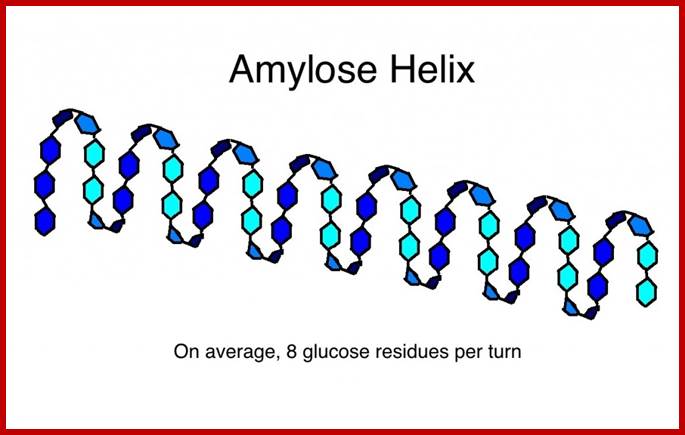
Eight glucose units per’turn; http://beerandwinejournal.com/
AMYLOPECTOSE
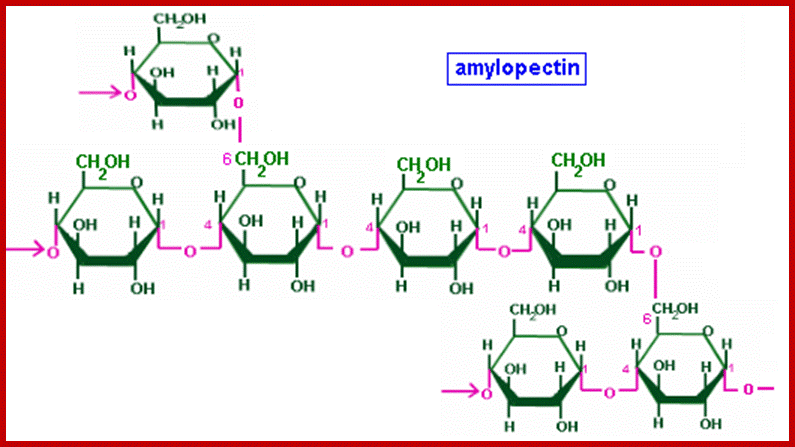
They are highly branched polymers of aD-glucose units. The backbone of the chain is made up of a number of glucose units held by aD1-4 glycosidic bonds similar to that of amyloses. But at the interval of every 24-30 glucose residues a branch develops due to ab1-6 linkages with glucose units found on the backbone chain. Amylopectins are very large and often reach colloidal dimensions, when dissolved in water the solution becomes viscous. In the presence of iodine amylopectins give deep violet color.
There is not much difference between plant starch and animal starch, where animal starch is called glycogen, except for the fact that the branching in glycogen is extensive. The aD1->6 branching is at the interval of every 8-10 glucose residues. X-ray diffraction patterns of amylopectins show that polymer chains are helically coiled to each other, similar to that of double helix. In fact, such chains are deposited in amyloplasts in concentric layers, which starts from a common centre called hilum. In many lower algae, starch is deposited around a proteinaceous body called pyrenoid found within chloroplasts. The deposition of starch layers is species specific, which is determined by its genetic factors.
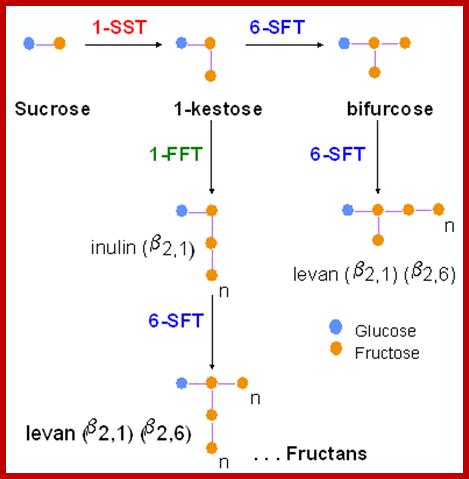
Apart from starch, there are other storage products such as polyfructans called levans, innulins, mannans, etc. Insulin is a polymer of D-fructose linked by β1->2 bonds. They are found in root tubers of Dahlias. Polymannans are found in yeasts, molds and some algae. Callose is a polymer of glucose units linked by aD1-3 bonds.
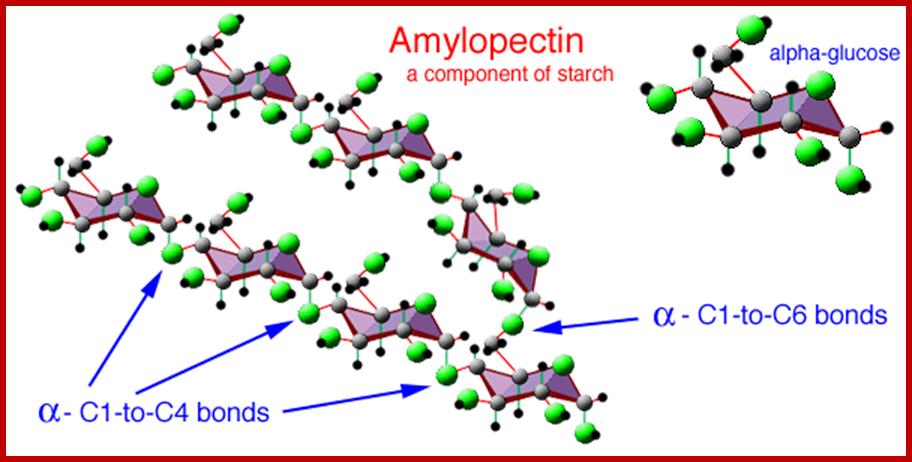
http://www.brooklyn.cuny.edu/
INTER CONVERSION OF CARBOHYDRATES
Synthesis and Degradation of Sucrose:
Sucrose is one of the most important natural organic substances that are used daily by mankind as a sweetening agent, but sucrose as a nutrient, has its own caloric value. But in recent years, non-caloric sweeteners have been found; e.g. Saccharin and monellin. Saccharin is 400 times sweeter than sucrose, monellin extracted from an African serendipity berry is 2000 times sweeter than sucrose and monellin happens to be a protein (into 11,000). Its sweetness has been attributed to its 3-D conformation of its polypeptide chain. When monellin is heated to denaturation, it looses its sweetness. Such non caloric sweeteners are very useful for diabetic patients. However, sucrose is a natural compound synthesized and stored in various organs of different plants, like sugarcane, sugar beet, sweet potato, etc. In fact plants, transport their photosynthate from the region of synthesis to the region of need in the form of sucrose or sucrose phosphates.
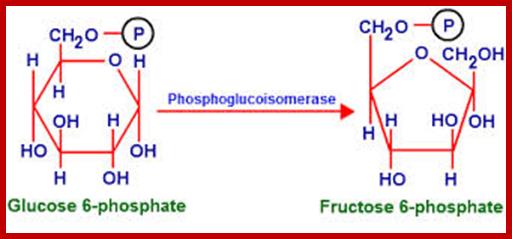
Though glucose and fructose are the components of sucrose, the synthesis of sucrose requires uridine diphosphate a-D glucose (UDPG) and β-fructose 6-phosphate. The condensation reaction is performed by sucrose phosphate synthetase. The sucrose 6-phosphate is then dephosphorylatated by sucrose 6-phosphotase enzyme.
However, the major pathway that operates in many plants is actually the reverse of sucrose breakdown. This method involves the condensation of UDPG and fructose directly. The enzyme involved in this reaction in sucrose synthetase. On the other hand, sucrose is hydrolyzed by sucrose phosphorylase or invertase.
SYNTHESIS OF STARCH
Plants synthesize starch as the major reserve food product in seeds and tubers. In lower plants, the synthesis and accumulation of starch takes place around pyrenoids found in chloroplasts. In higher plants though there is no such localization, starch is generally synthesized in chloroplasts or in amyloplasts, but Amyloplasts store starch as the major stored polysaccharide. The mechanism of starch synthesis has been studied extensively in the fruits of Zea mays and Oryza sativa and in the leaves of spinach, etc. In the above said plants it has been demonstrated that the starch synthesis is medicated by an enzyme called starch syntheses which exists in two isozyme forms. One utilizes ADP-Glucose to start with and the other uses Uridine-Di Phospho Glucose (UDPG). Though they require a primer (a short chain of glycans) for their activities, they can still perform polymerization of glucoses residues without them for they can prime such reactions.
Under normal cellular conditions the enzyme adds ADPG or UDPG to aD glucose present at the non reducing end of the primer and forms a1-4 glycosidic bonds. By this method, the enzyme goes on adding glucose units one after another, which results in the formation of an unbranched chain of glucose units called amylose. The synthesis of amylopectins also takes place along with the synthesis of amylose. The enzyme responsible for the branch formation is called Q-enzyme which is similar to that of branching enzyme found in animal systems. The Q-enzyme, isolated from starch containing tubers, has the ability to form a 1-4 as well as a 1-6 glycosidic bonds, simultaneously because both the enzyme forms are tightly bound to each other. However, recent studies on the nature of waxy and sweet corn mutants in Zea mays show that the waxy variety has greater amount of amylopectoses. The above observations suggest that there are to different enzymes for the synthesis of Amylose and Amylopectin and their activity is also independent of each other.
Plants also possess another pathway where amylose chains are synthesized by the activity of starch phosphorylase enzyme, which uses glucose 1, P and the same are added to the non reducing end of the primer chain. Thus the amylose chain grows. Cory and Cory have done extensive studies on these enzymes in animal systems. It is now almost accepted that the starch phosphorylase enzyme found in plants is similar to that of animal enzymes with minor variations; the activity of this enzyme is regulated by the levels of inorganic phosphates and some phytohormones.
DEGRADATION OF STARCH
In plant cells starch is degraded by the activities of a amylase, β amylase and starch phosphorylase enzymes. The activities of these enzymes may be independent or cumulative. Germinating seeds show very high activity of the above mentioned enzymes, but such enzymes are also found in the mesophyll cells of leaves.
The activity of starch phosphorylase enzyme is observed in both animal and plant cells; where amylose chains are hydrolyzed from the non reducing ends. The remarkable feature of these enzymes is that it utilizes inorganic phosphates and the same is added to glucose to yield glucose phosphate units. Such enzymes are also very active in guard cells during the opening of stomata. However, starch phosphorylase is incapable of degrading branching units.
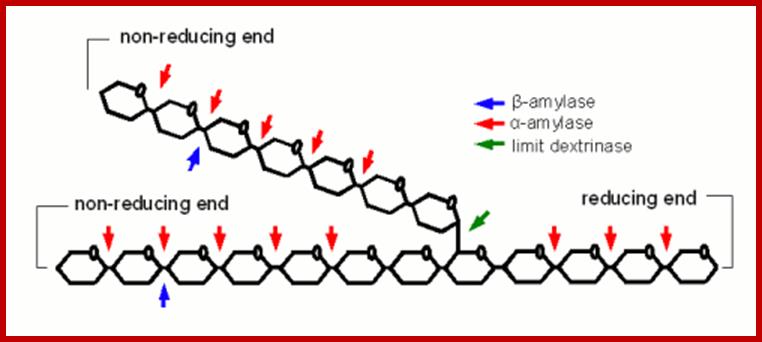
A large branched dextrin that shows which links can be broken by which of the mash enzymes; http://braukaiser.com/wiki/index.
Hydrolysis of starch by the activity of amylase enzymes is very interesting. While a amylase enzyme hydrolyzes chains randomly releasing single glucose units or maltoses, β-amylases hydrolyze amylose chains from non–reducing ends and the products are always maltose units. These enzymes are incapable of breaking the α1-6 branch chains. However, there are specific debranching enzymes called R-enzymes. They break a 1-6 bonds and thus complete the breakdown of starch. Later maltoses are also degraded into glucoses by the activity of maltase enzymes.
Germination of cereal grains results in the synthesis of a-amylase enzyme denovo. The synthesis of these enzymes is under the control of Gibberillic acid induced gene activation. The GA induced synthesis of a-amylase can be inhibited by the inhibitors like actinomycin D and cycloheximide, which inhibit transcription and translation respectively. The site of synthesis of the above enzyme is aleurone cells. The GA Produced by embryos diffuses into aleurone cells, where specific genes are activated. The resultant products like a amylases are secreted into endospermous tissue where the starch gets degraded into glucose and the same is used up by the growing embryo. The endosperm is a dead tissue. This work has generated a lot of interest in understanding how other hydrolyzing enzyme activities are regulated during germination and the development of plant body.
Inulin is another reserve food material found in the tubers of artichoke, dahlia, chicory and other composites members. It is made up of an unbranched chain of 35 fructose units linked by β 2-1 glycosidic bonds. Interestingly, the Inulin chain incorporates one molecule of glucose in the middle or at one end f the chain. The enzymes responsible for the synthesis and degradation of Inulin have been detected in many tubers containing Inulin as the reserve food material.
SYNTHESIS AND DEGRADATION OF CELL WALL COMPOUNDS
Cellulose is an important component of the cell walls. It is a polymer of β-D glucose units linked by β 1-4 glycosidic bonds. Cellulose and other cell wall carbohydrates form the bulk of the human diet. Unfortunately human beings are incapable of digesting cellulose and other related cell wall materials. Thus, in terms of calorie value, cellulose is useless as human diet. But for grass grazing animals it is the staple food, for they have the ability digest the cellulose and hemicelluloses in the intestine by the colon bacteria they have. Can you imagine, if human beings are introduced with such harmless bacteria, we can live on grass; world population does not and need not suffer from food shortage.
SYNTHESIS
The synthesis of cellulose has been studied under in vivo and invitro conditions in many systems such as mung bean, lupinus, pea plants and also in some bacteria. Though βD-glucoses are the main precursors, even mannose, galactose and other hexoses are utilized in the cell wall formation by converting them into glucoses. The enzyme called βD-1-4 glyconyl transferase which is also called cellulose synthase, is responsible for the synthesis of cellulose. The enzyme utilizes GDP glucose or UDP glucose as the free units, and the same are linked to cellobiose units by the formation of βD1-4 glycosidic bonds. Here cellobiose units act as primers. Thus a long chain of cellulose fibers develop. The cellulose synthesizing enzymes are located in the membrane fractions of the plant cells. In fact, Golgi vesicles get loaded with enzymes and the precursor hexoses, and then they are transported to the plasma membrane where the vesicles by exocytosis secrete the enzymes and cell wall components. The transportation of Golgi vesicles is greatly aided by microtubules found in the cytoplasm. In recent years attempts to synthesize pure cellulose fibrils in test tubes have met with great success. Perhaps in another decade or so, cellulose fibers needed for cloth making will be artificially produced in industries with the use of sophisticated bio-techniques.
DEGRADATION
Cellulose is the primary component of primary wall, but secondary walls are made up of cellulose, hemicellulose, lignin and other components. However, cellulose is degraded by the activity of cellulase enzyme which exists in two forms. One is active at acidic pH i.e. 4.5 to 5.0 and the other form is active at pH 5.9 to 6.3. They are located at the outer surface of the plasma membranes. These enzymes hydrolyze the B 1.-4 glycosidic bonds randomly and produce disaccharides or small oligosaccharides called cellobiose. Then cellobiase enzymes break down collobioses into glucose units.
The synthesis and activity of cellulase enzymes, however is under the control of phytohormones like auxins. During auxin induced cell elongation the acidic form of cellulases becomes active and the cell wall is loosened by hydrolytic activity. In addition, auxin induced cellulase synthesis by specific gene activation. Similarly, ethylene is another hormone which induces the synthesis of cellulase and pectinase denovo during fruit ripening. Even Abscisic acid induces cellulase synthesis during abscission layer formation in the stalks of leaves and fruits. The synthesis and degradation of other cell wall components like Polymannans, polyxylans, polyarabons, lignin and other pectic components have been observed and they are found to be under gene regulation.
CENTRAL ROLE OF GLUCOSE
Glucose plays a central role in the inter conversions of simple monosaccharides into various polysaccharides and vice versa. Interconversions are highly regulated. This depends upon the metabolic state or the needs of cells. High energy nucleotides like ATP, GTP and UTP are absolutely necessary in the synthetic process of carbohydrates. Further more glucose is oxidized to produce energy rich compounds. Sometimes many intermediary carbon compounds, required for the synthesis of many amino acids and other organic compounds are also produced. Under certain conditions, amino acids and other components are utilized in the synthesis of glucoses by the reverse pathway of glycolysis to produce glucose, which is known to be the ultimate product of photosynthesis, where solar energy is stored in the form of chemical energy (in chemical bonds) not only provides chemical energy but also acts as the structural components for many cell structures.