APOPTOSIS 1
Introduction
Cell Proliferation and Apoptosis:
Repeated cell division and programmed cell differentiation is responsible for the development of a multicellular organism. Cell cycle events are themselves are programmed and genetically regulated. Pronounce, Apoptosis as Apo-Ptosis not as Apoptosis. In older organisms cells in tissues die for various reasons, one is injury that leads to death of cells by what is called necrosis. But certain injury at molecular level is deadly in the sense it can cause injury to other nearest neighboring cells, so cells have inbuilt sensing mechanism by which they initiate programmed cell death. If there is any loss of cells in a given tissue, for cells replacement, quiescent cells get activated to divide and redivide till the lost number of cells is averaged out. Exception to this is neuronal cells. Death of neuronal cells is permanent, and they are not replaced as in other tissues. However, it is now noticed that stem cells might be able become neuron cells?! Multipotent neuronal stem cells (those that can generate different types of cells) are present in these regions, and all of these cells differentiate into neural cell forms, such as neurons, oligodendrocytes and astrocytes. Death comes to cells in different forms so different names:
Necrosis: Cells get injured, cells get punctured where cells lyse extruding various injurious components, which cause severe damage to other neighboring cells, causing a widespread destruction; this is like carnage.

Necrosis caused by H2OF2 emitted from an aluminum plant-grape.www.pbs.org

Necrosis spreading from the margins to areas between the main veins (J. O'Sullivan). http://keys.lucidcentral.org/
Paraptosis: Cells swell, develop large bubbles or vacuoles with liquid inside and die; this method of suicide is called parapoptosis. They don’t employ Caspases, which is hallmark of apoptosis. This method or most similar methods have been observed in yeast cells.

Diagram of cellular differences between various pathways; en.wikipedia.org
Autoschizis: It is a bizarre type of death; it is a novel type of programmed cell death. Cells develop crates inside and cell organelles escape from the cell and they are destroyed by some proteases that develop inside the cell. This happens when cancerous cells are treated with Vitamin-C and K. sub3. Normal cells remain unaffected, but many cells die because induced apoptosis, but substantial number of cancer cells die by autoschizis. Autoschizis is a novel type of programmed cell death characterized by exaggerated membrane damage and the progressive loss of cytoplasm through a series of self-excisions. These self-excisions typically continue until the perikaryon consists of an apparently intact, round nucleus surrounded by a thin rim of cytoplasm that contains damaged organelles. During the process of cell death by autoschizis, nucleoplasm, initially become more chromatic and then progressively loses chromaticity as their size decreases. Concomitant with this diminution in cell size, the nuclei become smaller and contain large nucleoli, which become round and compact. Therefore, before it dies, the size of the resultant autoschizic cell is much smaller than the tumor cell from which it originated.

www.autoschizis.com; www.pharma-board.com


The figure is self-explanatory, where cells going through fragmentation with blebs and secondary necrosis; clinicalscienceblogtokelo.wordpress.com
Oncosis: Cells expand by taking in lot of water in an uncontrolled manner. Soon proteins become denatured like cooked yolk proteins, then cells take excess calcium into cells and death follows.

This is due to a differential distribution of proteins on either side of the impermeable inner mitochondrial membrane. In Oncosis the loss of Δψm is dramatic compared to the loss observed during apoptosis. This observation is reflected in the energy demands of Oncosis and apoptosis upon the cell, Oncosis being ATP independent and does not require mitochondrial function to proceed. Whilst apoptosis is ATP dependent but requires a degree of mitochondrial function during the apoptotic process.
Apoptosis: Programmed cell death has certain morphological manifestations in contrast to cell death by necrosis and others mentioned above. On the contrary, Apoptosis or what is called-programmed cell death, is stimulated by certain factors and it leads to DNA fragmentation of 180 base pairs long (characteristic of apoptosis), intracellular organelles undergo fragmentation including nucleus and the cell collapses producing blebs and membranous vesicles pop out of the cell surface. Most of these vesicles contain cellular components. When they are budded off they are recognized and engulfed by macrophages and consumed.

Figure shows plant cell death in leaves marked by their dried nature;

Figure demonstrates cellular changes and the fragmentation of DNA; wordpress.com712 × 507 intranet.tdmu.edu.ua.


Cells on the left are growing in response to mitogens, but on the right-side cells are in course death in response to death signals. www.utm.utoronto.ca

aquamarin.kg7.ru; voda.vsesekreti.net
Development is genetically programmed and it goes through step by step and passes through various stages. During developmental stages, however, in a variety of animal and plant species, fully developed cells do undergo cell death.
· In plants, cell death is perfected in such a way, they leave structurally modified cells dead, but intact for specific functions, example most of the Xylem elements and sclerenchyma elements go through such process. But developed cells remain intact and functional.
· In developing tadpole its tail is resorbed by apoptosis; the gradual degradation is not uninhibited invasive, once reaches a point, it stops.
· During human embryo development, the web like structure in between the fingers is gradually degraded by programmed cell death. Regression of human embryonic tail, nictitating membrane
· In the case of development of the nematode worm of Caenorhabditis elegans, cell divisions and differentiation lead to the formation of 1090 cells, of which 130 (1) cells die in a manner called programmed death of those cells only which are marked. This is an essential part of the development of this organism. It is not restricted to the worm; this phenomenon is spread over almost all multicellular organisms.
· Many neuronal and immune cells die a programmed cell death.
· In drosophila expression of rpr gene marks the cell for death. In moth during transition from pupa to insect, most of the muscle cells die. This death induced by the lack of ecdysone. The cell death can be rescued by treatment with ActinomycinD-D.

Genotoxic agents or inflammatory cytokines activate cellular stress responses and trigger programmed cell death. We have identified a signal transduction module, including three nuclear proteins that participate in the regulation of cell death induced by chemotherapeutic agents and tumor necrosis factor (TNF). In this nuclear signaling module, retinoblastoma protein (Rb) functions as an inhibitor of apoptotic signal transduction. Inactivation of Rb by phosphorylation or caspase-dependent cleavage/degradation is required for cell death to occur. Rb inhibits the Abl tyrosine kinase. Thus, Rb inactivation is a pre-requisite for Abl activation by DNA damage or TNF. Activation of nuclear Abl and its downstream effector p73 induces mitochondria dependent cell death. The involvement of these nuclear signal transducers in TNF induced apoptosis, which does not require new gene expression, indicates that nuclear events other than transcription can contribute to extrinsic apoptotic signal transduction. Cellular response to death signals leading to death; Jean Y J WANG http://www.nature.com/
· Inhibitor of gene expression at transcription level, which suggests that death requires gene expression.
· In Munducta, Antherasa and Polyphemus (giant silk worm) during transition polyubiquitin is expressed, and this targets protein for proteasome-mediated destruction.
· Cell death can also be due to cytotoxic T-cell, which attack marked cells and destroy by Granzymes. For example, HIV infected cells do die by apoptotic process.
· The programmed cell death is predictable, defined in time and place. Death by this way is not passive but an active process involving gene activation.
· Dead cell can be detected by Acridine orange or Tryptan blue staining methods. Tryptan blue stains only dead cells. One can also use Propidium iodide, which is a DNA dye, which excludes dead cells. Cell death can also be monitored by the increased activity of polyubiquitylation, increased activity of calcium dependent endonuclease activity, which cuts DNA into 180bplong fragments.
· Expression of receptors with death domain at cell surface is another determinant of death process.

Apoptosis activated by Intrinsic and extrinsic pathway; pt851.wikidot.com
Intrinsic pathway or mitochondrial pathway;
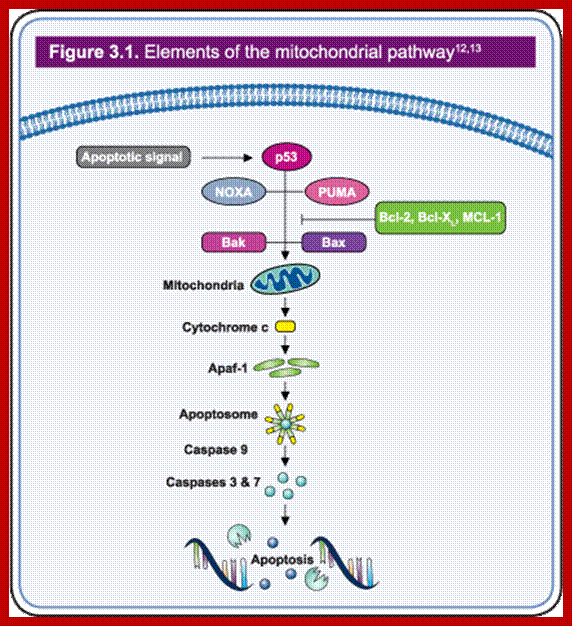
Intrinsic pathway or mitochondrial pathway; http://www.biooncology.com/

Elements of the extrinsic apoptotic pathway; http://www.biooncology.com/

Extrinsic and Intrinsic pathways; www.scq.ubc.ca

Apoptosis
is triggered through 2 main signaling pathways; http://cancercelltreatment.com/
The extrinsic pathway, which is activated in response to multiple external pro-apoptotic signals, including endogenous Apo2L/TRAIL and other pro-apoptotic receptor agonists (PARAs)
The intrinsic pathway, which is activated by cellular developmental cues or as a result of severe cellular stress (eg, DNA damage). www.biooncology.com

This pathway is often activated in response to signals resulting from DNA damage, loss of cell-survival factors, or other types of severe cell stress. Normally, pro-apoptotic proteins are released from the mitochondria to activate caspase proteases and trigger apoptosis. The intrinsic pathway hinges on the balance of activity between pro- and anti-apoptotic signals of the Bcl-2 family. Preclinical studies indicate that members of the Bcl-2 family regulate the permeability of the mitochondrial membrane and determine whether a pro- or anti-apoptotic signal will be released inside the cell.
· The extrinsic pathway begins outside the cell through activation of pro-apoptotic receptors on the cell surface. These are activated by molecules known as pro-apoptotic ligands.
· Preclinical studies show that ligand binding causes receptors to cluster and ultimately form a death-inducing signaling complex (DISC).
· Upon DISC activation, the extrinsic pathway has been seen to adopt the same effector caspase machinery as the intrinsic pathway.
Apoptic signals:
· The most common factor for cells to induce apoptosis is lack of essential growth factors.
· Ecdocyne deficiency in Moth.
· The Tumor Necrosis Factor (TNF) family comprises several ligands, such as the prototype TNF-, the Fas Ligand (Fas) and TNF-Related Apoptosis-Inducing Ligand (TRAIL/Apo2L).
· Thymocyte cells treated with glucocorticoids.
· Androgens in prostate cancer cells.
· Cells exposed gamma irradiation.
· Often cells are marked, example cytotoxic T-cells attack and kill target cells, which are immunologically marked.
· Irreparable damage to DNA. Internal factors such as Myc and p53 and its associated inhibitor proteins respond to such damages and initiated death.
· Any other stress factors can induce cell death; this amounts to molecular insult to the cell; “when you are abused to such an extent, what is the use of living, so commit suicide to avoid the insult”.
· Some of the ligands like Fas-L and TNF a 1 are expressed as membrane bound proteins, but they are cleaved and released as soluble forms. Soluble forms of ligands are mostly produced by macrophages. Some of the Tumor Necrosis factors (TNFs) produced are pleiotropic forms and they elicit many cellular responses through the binding to their respective cellular receptors.
|
Receptor |
Ligands |
Adaptors |
Targets |
|
|
Fas/CD95 Apo1 |
Fas-L Apo-IL |
FADD/ MORT1 |
Pro casp 8, |
Apoptosis |
|
TNF-R1 |
TNF-alfa |
TRADD, FADD |
Pro-casp 8 |
Apoptosis |
|
TN-R1 |
TNF-alfa |
TRDD/RIP/TRAF2 |
MEKK, jun/Ap1 NFB |
Cell proliferation inflammation |
|
TNFR2 |
TNF alfa |
TRAF2/ TRF1 |
MEKK,IKK,NFkB |
Cell proliferation inflammation |
|
DR3,4,5 |
Trail APO-2L |
FADD |
Pro cas8 |
Apoptosis |
|
DCR1-3 |
TRAIL/APO2L |
None |
Decoy receptor |
Ligand sequestration |
TRDD= TNF receptor associate death domain.
RIP= Receptor interacting protein.
TRAF = TNF receptor associated factor.
TRAIL = TNF related apoptosis inducing ligands.
Which cells are marked for apoptosis?
1. Cells treated with carcinogenic chemicals, which express death signals.
2. Cells, which have no function. If the cells are in excess than required they are subjected to death.
3. Cells that develop at improper places and at improper time they are induced for death.
4. Cells, which have completed their function and those having very harmful effect on other cells, are marked for death.
Effect of excess apoptosis:
Many a times excess apoptosis takes place for wrong reasons, in such cases certain diseases develop, such as–insulitis, hepatitis, allergic encephalitis, Alzheimer disease, ischemic disease and many others.
Genes and the mechanism of apoptosis are discussed in the next chapter.
Apoptosis in Plants (A general account): Apoptosis in plants: specific features of plant apoptotic cells and effect of various factors and agents.
Vanyushin BF1, Bakeeva LE, Zamyatnina VA, Aleksandrushkina NI.; Author information
Abstract
Apoptosis is an integral part of plant ontogenesis; it is controlled by cellular oxidative status, phytohormones, and DNA methylation. In wheat plants apoptosis appears at early stages of development in coleoptile and initial leaf of 5- to 6-day-old seedlings. Distinct ultra-structural features of apoptosis observed are (1). Compaction and vacuolization of cytoplasm in the apoptotic cell, (2). Specific fragmentation of cytoplasm and appearance in the vacuole of unique single-membrane vesicles containing active organelles ( 3). Cessation of nuclear DNA synthesis (4). Condensation and margination of chromatin in the nucleus, (5). Internucleosomal fragmentation of nuclear DNA and (6). Intense synthesis of mitochondrial DNA in vacuolar vesicles.
Peroxides, Abscisic acid, ethylene releaser ethrel, and DNA methylation inhibitor 5-azacytidine induce and stimulate apoptosis. Modulation of the reactive oxygen species (ROS) level in seedling by antioxidants and peroxides results in tissue-specific changes in the target date for the appearance and the intensity of apoptosis. Antioxidant butylated hydroxytoluene (BHT) reduces the amount of ROS and prevents apoptosis in etiolated seedlings, prolongs coleoptile life span, and prevents the appearance of all apoptotic features mentioned. Besides, BHT induces large structural changes in the organization of all cellular organelles and the formation of new unusual membrane structures in the cytoplasm. BHT distorts mitosis and these results in the appearance of multiblade polyploid nuclei and multinuclear cells. In roots of etiolated wheat seedlings, BHT induces differentiation of plastids with the formation of chloro (chromo) plasts. Therefore, ROS controlled by BHT seems to regulate mitosis, trigger apoptosis, and control plastid differentiation and the organization of various cellular structures formed by endocytoplasmic reticulum.
Apoptosis’ term derived from Greek word- falling of leaves from the tree,
Which cells are killed by PCD:
Detection of dead cells:
DAPI –by Hoechst company- 5’6-diamino-2-phenylindole-dead cells take stain- fluorescent,
Annexin –V fluorescent dye that binds to Phosphatidyl serine found on cell surface of apoptotic cells.
TUNNEL- Fragmented DNA can be labeled at 3’end by Terminal dUTP transferase mediated labeling,
Electrophoresis of cell DNA- shows a ladder of 180bp fragments-characteristic.
Propidium iodide, binds to DNA of living cells but not to dead cells.
Which cells Die and why?
Cell death is maintained when there is excess of cells produced than required,
Cells that have lost their viability and function or damaged cells which are not functional; Cell types found in wrong place are put to PCD. Nerve cells- which are not interconnected; in cat 80% of the retinal cells die.
Billions of cells die in bone marrow- not activated and differentiated.
Structures get cell death- Vertebral tail, regeneration of Tadpole tail, gills,
web between fingers during child development in mothers’ womb in humans.
In C. elegans out of 1090 cells 131 cells die during development of Ulva and other by PCD, this is a must. Drosophila-Larva to pupa and pupa to insect a large section of cells dies.
PCD in plant cells is more or less autophagic, similar to yeast when they are subjected glucose repression. Death of cells occurs during abscission of falling of leaves, corolla, and transformation of young Xylem elements into Trachea and tracheid’s and sclerenchymatous fibers. In trachieds vacuoles develop and bursts open to clear all the mess. Tracheid’s require for the transport of water and minerals and sclerenchymatous cells require for mechanical strength.
Mesophyll cells under in vitro conditions induced to form trachieds die by PCD.
Hypersensitive reaction due to viral infection induce cell death, response to infection involves ion fluxes, calcium influx, generate reactive oxygen radicals, produce nitric oxide, activate MAPK.
PCD is observed in or during-
Megaspore formation,
During the death of antipodal and synergid cells,
Endosperm formation (endosperm cells are dead cells),
Trichomes,
During the development of sclerenchyma, Xylem elements, root cap,
Leaf senescence,
Suspensor degeneration,
Unfertilized flowers,
After fertilization-stamens, petals,
Unisexual flowers and their parts,
In Zea mays tassels contain both male and female flowers, but females die, but in tassel mutant2- female cells wont die, so female flowers require Tassel2 gene for it death.
Megaspore formation -3 cells die,
In anther, tappetum dies slowly,
During development of Zygote the upper thee cells die and the lower the lower with fusion of male nucleus develops into an embryo.
In Monstera (Swiss cheese plant) death of cells leads to indentations,
In roots hypoxia leads to cell death and create air cavities-aerenchyma.
Most of the deciduous plant’s leaves fall by PCD, but some plants retain leaves up to 45 years ex. Prunus longaevia found living at 5066 yrs old, Welwitschia mirabilis (bristle cone pine).
Immune cells- which are not activated; Wolffian ducts in Females –gonadal cells migrate to the ovary at 5th and 6th week of the embryo; multiply later cells die large numbers, all oocytes mature to diplotene stage and stay put till the female comes to heat- in the course of time thousands of oocytes die by apoptosis. If active a female can release 600-700 cells productive cells.
Mullarial cells in males- germ line cells migrate and renew as and when they are exhausted.
Components or signals that induce Apoptosis:
Genotoxic compounds- reactive oxygen ions (ROS).
H2O2.
(-) cytokines,
Heat shock,
Fas L,
TNF alpha,
Radiation,
Cytotoxic drugs,
Antimicrobial drugs,
Infection with certain viruses,
Activation of p53,
Inactivation of gene expression by Actinomycin-D,
Arachidonyl tri fluromethyl ketene,
Staurosporine,
Sodium salicylate,
C6- ceramides.
Unregulated Apoptosis:
Autoimmune disease can lead to large number of cell death. Ischemic injury to vascular epithelial tissue, neuron degenerative, Alzheimer disease, Parkinson disease, Huntington chorea, spinal muscular atrophy, demyelating neuropathy, viruses induce cell death-HIV, Ebola, parasites like trypanosomes-death of T-Cells.
Death target:
All cytoskeleton elements, nuclear lamins, nuclear DNA and all cellular proteins die. Plant senescence is associated genes and proteins have been identified, at least fifty of them are known from-Arabidopsis thaliana, Brassica, Maize, Cucumber, Asparagus, Yomato, Rice, Barley, Lolium, Banana, Melons and many others.
The nomenclature of plant apoptosis or senescence genes is slightly different from animal systems.
Arabidopsis SAG2, 12- Cys-protease,
Maize Sec1, 2- Cys-protease,
Brassica napus- LSC 7, 90- Cys-protease,
Brassica napus Lsc60 Aspartic protease,
Nicotiana UBC4, ubiquitin carrier,
Potato UB17 polyubiquitin,
Arabidopsis RNS2 ribonuclease,
Cucumber MS malate synthase,
Cucumber IC2 isocitrate lyase,
B.napus LSC54 metallothioninI,
B.napus LSC650 catalase,
Tomato Tom13 ACC oxidase
In plants senescence, first set of enzymes that express are metabolic converters, then second set of genes express are responsible for senescence.
Mendel’s green pea cotyledons are due to mutant gene called B or now called- I. Mutation causes delayed senescence. Recessive –I, lack chlorophyll degrading enzyme called Pheophorbide a-oxygenease (PaO).
Fruit ripening tomato requires ACC oxidase and 1-amino cyclopropane 1-carboxilic acid synthase.
GA induces aleurone cells death by inducing autolysis, vacuolation and collapse of protoplasm, this happens when the food is mobilized out. ABA prevents aleurone cells death or postpones PCD.
GA induced PCD involves signaling cascade such as cytosolic Ca2+, increase in cytosolic pH, increase cGMP, CAM and protein kinases and protein phosphotases
Oxygen depravation induces aerenchyma formation – due to induction of Xyloglycan Endo Trans glycosidase (XET), which causes the degradation of cellular cytoplasmic components.
Pathogens cause hypersensitivity that causes cell death. Disease resistant genes called R act on AVR genes of pathogens when R (-) and AVR (-) plants are susceptible to pathogens. Pathogen invasion results in the production of highly reactive oxygen intermediates and super oxides are produced, that induces cell death.
O2->e->O2^-àSODà H2O2à OH*à H2O
HO2*=hydro peroxide,
O2*(-) = super oxide anion,
O2 + H+ à HO2* hydro peroxides.
Pathogen infection leads to Hypersensitivity reaction and induce PCD, which can be observed as apoptotic bodies such as blebbing, nuclear membrane degradation, DNA cleavage to 50KB and 180bp pieces.