Genetic Engineering:
Application-Animal Biotechnology:
Transgenic animals:
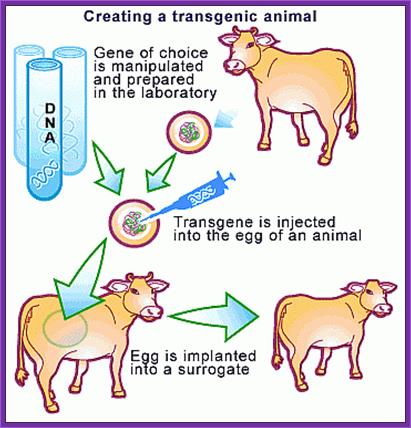
Ever since the modern human specie arrived on the surface of this planet and started walking around and started living in groups, he collected animals and plants of his liking for his own use. He also reared them and stock piled them under his care. This is the beginning of animal and plant farming. He also observed variation among the same animal and plants; and cross bred them to develop new varieties, this is yet another technology innovated by him called reproductive breeding, where he used transfer of an entire genome from one to another. This is nothing short of “Transgenic” methodology. In 20th century one uses techniques where one or two genes are transferred into animal cells and obtain a complete animal called transgenic animals; so also transgenic plants were obtained.
In fact animal cloning experiments started early 1960s and by sixties experimenters were able to get a large number of amphibian clones. The technique was simple; frog eggs were laid outside the body. The eggs were large enough to be seen by naked eye. One can obtain such eggs in large numbers whenever one like by injecting frogs with reproductive hormones. Enucleation of eggs was a simple technique, which was perfected at that time. Then 2n nucleus sucked from somatic cells such as intestine or active cells, and injected the same into enucleated eggs and prodded the cells to grow; and actually they grew into normal frogs. Several hundreds of such amphibians were grown for experimental purposes. In fact fishes were also used for the same purpose. Some people have perfected culture medium for regrowth of organs such as frog palm or limbs by using special culture medium containing Vitamin-A. Today in vitro culture of pancreatic cells has been successful. Several tissue cells have been grown in in vitro conditions and they are used for variety of experiment purpose.
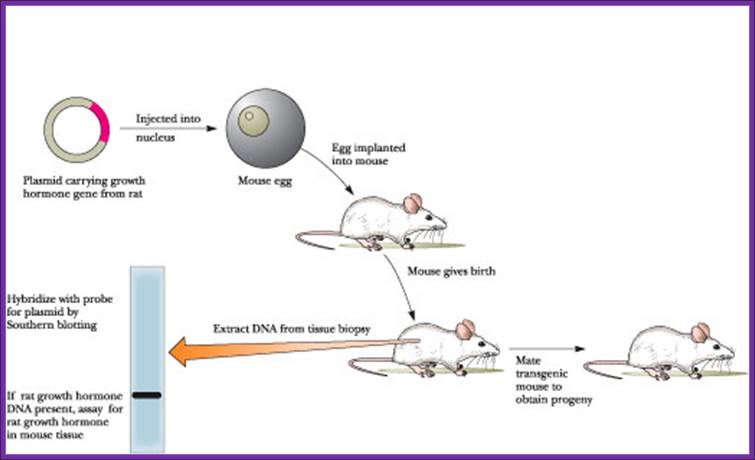
The first transgenic animal produced was from Ralph Brienster, in Lippincott, Univ.of Penn, Philadelphia, in 1980-82. They introduced human growth hormone gene into mice eggs.
Donor strain mice were reared and females were injected with pregnant mare serum, or one can use porcine follicle stimulating hormone. After 48 hrs human Gonadotrophin was injected. Hybrid mice are better than inbreed mice?
Such females were mated with selected male studs. Each of the female will produce 18-20 fertilized eggs. After 12 hrs of post coitum, oviducts were taken out and the eggs squeezed out into specific nutrient M16 media. Wash eggs in M16 media and then they are transferred to M2 media micro drops.
M16 Media:
NaCl,
KCl,
KH2PO4,
MgSO4,
Glucose,
NaHCO3,
Na Pyruvate,
CaCl2,
HEPES, pH 7.5,
Phenol Red.

http://bsoha.com/

Microinjection Equipment-http://www.en.wikipedia.org
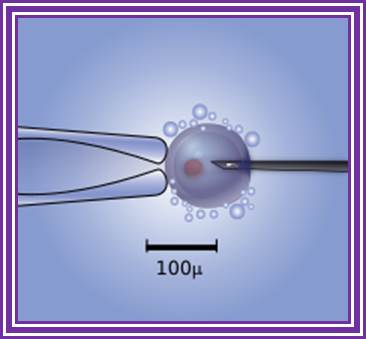
Diagram of the intra-cytoplasmic sperm injection to a human egg. Micromanipulator on the left holds egg in position while microinjector on the right delivers a single sperm cell. http://www.wikipedia.org
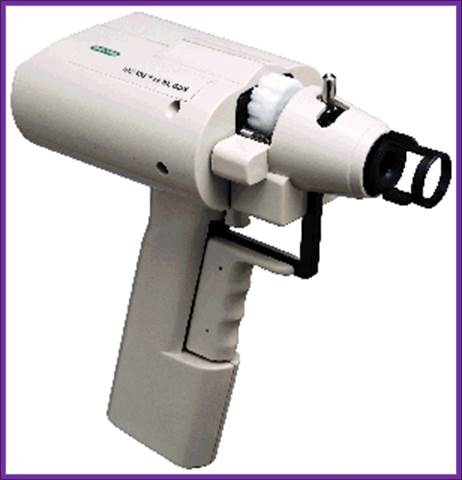
Gene gun used to bombard DNA into plant leaf cells;
The instrument used for microinjection of DNA into the plant cells http://www.bio.davidson.edu/

Gene gun is more useful in injecting DNA into plants cells. Natural News Blog;; http: //naturalnewsblog.blogspot.com/
Use powerful dissecting microscope equipped with microinjecting facility having very thin and narrow glass tips which can hold only few μl of liquid.
Inject linearized plasmid DNA containing Growth hormone gene into the nucleus of the egg.
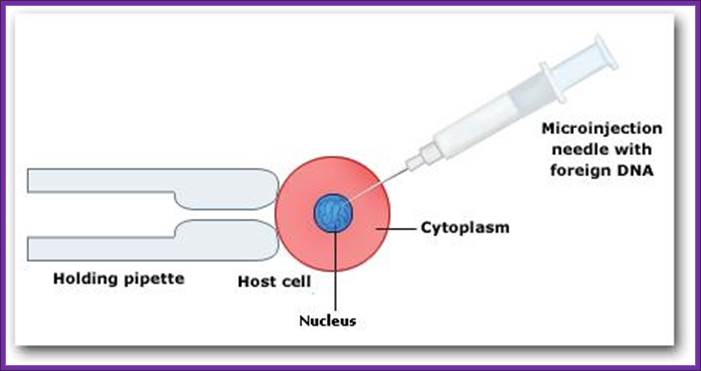
Microinjection of DNA into the nucleus, using pipettes genetically modified organisms; http://www.hwdsb.onca
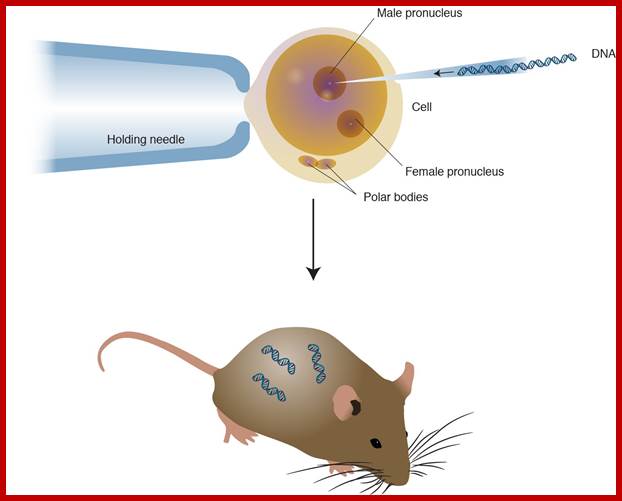
Modified Genetic RDNA or engineered DNA is injected into fertile egg and transplanted into frtile mice. Transgenic Organisms; http://knowgenetics.org/
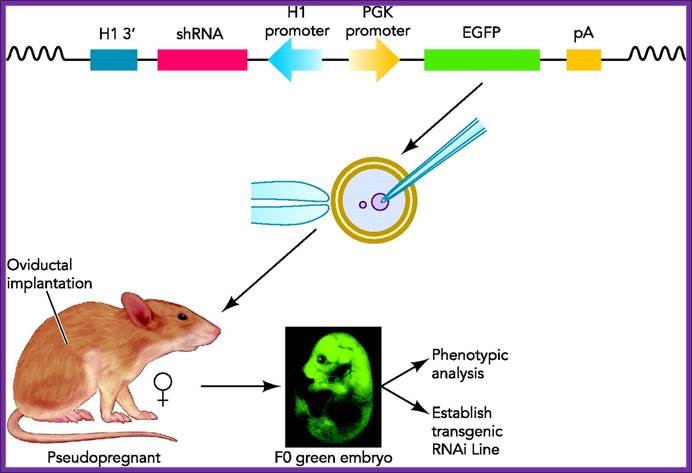
Transgenic RNAi not only allows the study of biological processes not present in cultured cells but also offers chronic therapeutic potentials. In this review, we will summarize the developments in the generation of transgenic RNAi mice. Transgenic RNA Interference in Mice;;http://physiologyonline.physiology.org/
The amount injected is about 2picoliters containing at least 200 copies.
Prepare the set of mice by injecting hormones and mated with vesectomiced males when the females are in estrous stage or what is called animal in “Heat”. These animals behave or rather feel as pseudo pregnant and act as foster mothers.
All these operations have to be done in sterile condition and the job requires skill and deft hands. Under anesthetic conditions these females are operated and the oviduct and uterus is pulled out. Then eggs injected with a gene are then transferred into the uterus via infundibulum. Stitch the cut ends of the animal and inject with antibiotics (heavy dose).
In about 19-22 days pups will be delivered. When they are 10 to 15 days old one can peal off tail skin, for it grows back, and homogenize and isolate DNA and do southern blot, or isolate RNA and do northern blot or isolate proteins and do western blot and find out whether or not the injected genes are integrated and expressed their gene products.
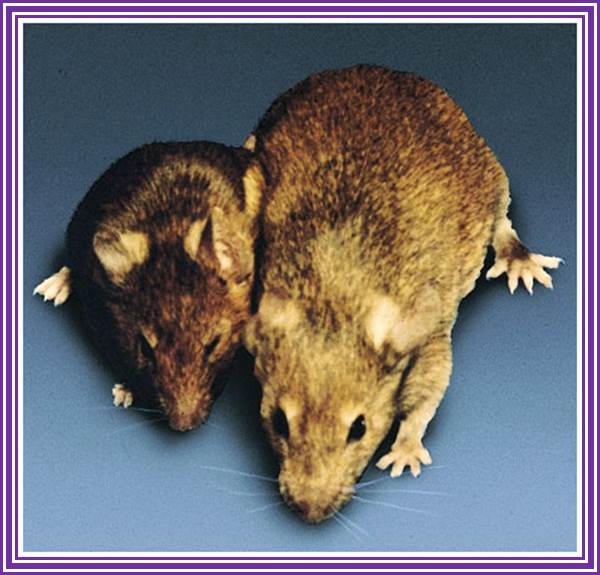


Brinster Mice007.jpg; Dr. Ralph Brienster first transgenic mices, produced in the lab (1980-81), Univ.Penn;
Dr.G.R.kantharaj, the author of this website a witness when these mices were born in the Univ. Penn, downtown campus. Dr Ralph Brienster is good friend of GRK https://www.avma.org
Transgenic animals can also be obtained by another method where blastocysts derived stem cells are transfected with the desirable gene placed under specific promoter to express under either induced condition or expressed in tissue specific manner. They are selected against an antibiotic.
They are further amplified and the same are transfused into freshly isolated blastocysts, which are then transplanted into uterus for further development. Many mice cell lines have been created in this manner. One such cell line is used for gene knock out experiment.
Molly and Polly were obtained as transgenic animals with the gene Factor-IX under the promoter beta lactoglobin for it expression in the mammary tissue during milking period. A thirty day fetal cell was used to produce “Transgenic Bull” in 1997 by ABC-Global at Wisconsin. In Honolulu scientists obtained cow.
Cloning word has larger implications in terms of technology, such as DNA cloning, Reproductive Animal cloning and therapeutical cloning. DNA cloning involves, cut the DNA and add a required promoter and select a specific sequence and ligate it to a vector. Animal cloning involves taking 2n fertilized egg, remove the nucleus and fuse with 2n embryonic cells or adult cells. Therapeutical cloning uses blastocysts or stem cells from different tissues and regenerate the tissue or the organ for transplantation.
Animal cloning was new technique for it was already achieved in 1960s; frogs were the first to be cloned animals. In later years scientists have shown new techniques employed in developing animal cloning. In fact any cell having the full complement of genome is endowed with potentiality to regrow into a complete animal if the cells are provided with proper media and the required vitamins and hormones and stimulus. This is what is called as totipotency of cells. This phenomenon is easily demonstrated plants, where the plants are grown from one or two nucleated cells. This technique of Micropropogation is now employed in agriculture and horticulture.
Professor Sir Ian Wilmut, Edinburgh, the person who led the team in the creation of Dolly the Sheep, They were the most celebrated animals that were obtained, in 1996-97, by modified cloning technique was a sheep called Dolly named after popular singer Dolly Parteon. British scientists (Keith Campbell) from Rosalyn institute had field days of glory and rejoiced in spite of some diehard critics. Dolly started her life, as with all other cloned animals, in a test tube. Once normal development was confirmed at 6 days, the embryo, that was eventually to become Dolly, was transferred into a surrogate mother. British scientists (Keith Campbell) from Rosalyn institute countered the knighthood awarded to Sir Ian Wilmut. When Sir Ian was asked directly he replied “I did not create Dolly" was true, Sir Ian replied "yes". He also said that Professor Campbell deserves 66 per cent of the credit for Dolly, although Sir Ian insisted on putting his own name at the beginning of the scientific paper published in the journal Nature in February 1997.
Pregnancy was confirmed by an ultrasound scanning at about 45 days of gestation and the pregnancy was monitored closely for the remaining 100 days. The pregnancy went without a problem and Dolly was born on the 5th July 1996. Unlike many cloned animals, which often have neonatal problems at birth, Dolly was a normal vigorous lamb and was standing and sucking unaided within minutes. The animal technicians were aware that this was an important lamb and critical to the research team that had produced her but they were completely unaware of the impact she would finally have. Another scientist to be honored is Debby Reynolds, the UK government's former chief veterinary officer. Dolly was born on 5th July, 1996 died at the age of six.

Animals Whose Genes Have Been Tinkered with by scientists; http://xfinity.comcast.net/

Dolly; http://www.sheep101.info/
Here on the ‘Towards Dolly’ team couldn’t let the 05 July go by without celebrating namesake, who was born on this day in 1996. To most people, Dolly the sheep (1996-2003) the first mammal to be cloned from adult cells, Dolly was produced at the Roslin Institute, Edinburgh as part of research into producing medicines in the milk of farm animals; Nature (385, 753-844) on 27 February 1997, http://libraryblogs.is.ed.ac.uk/ Dolly was named after singer Dolly Parton.
Americans were not far behind for they have also achieved animal cloning especially monkeys. They used different techniques.
Fusing enucleated egg cell with cultured udder epithelial cell from six-year-old female sheep at Go stage, with an electric shock. They have made 227 attempts before they were able to get this cloned sheep. Dolly has the distinction of becoming a mother. There are claims that they have used fetal stem cells.
There were some claims that the Dolly was obtained from fetal cells. Unfortunately, after all these years of glory, Dolly was believed to be suffered from arthritis. The disease Arthritis may be due to other reasons, not because it is a cloned animal. It died but left a progeny; Dolly's Family.

Dolly and her first lamb, Bonnie, born April 13th 1998 and fathered naturally by David, a welsh mountain Ram; Father of Dolly- awarded knight hood ; Dolly and Bonny; Dolly died on 14th Feb 2003; http://www.coldmeadow.com/
In an attempt to allow Dolly to have as normal as life as possible it was decided that she should be allowed to breed. A small welsh mountain ram was selected as her mate and between them they successfully produced 6 lambs. Their first, Bonny, was born in the spring of 1998. Twins followed the next year and triplets the year after that.

Dolly was made immortal by duplicating in the wax.

Professor Sir Ian Wilmut and Dolly the Sheep; Goat /sheep called Dolly?; http://www.ed.ac.uk/
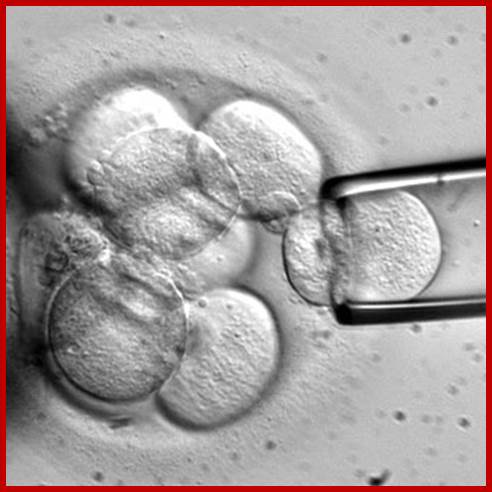
Monkeys were developed by growing an embryo to 8-16 cells and then the cells were separated and grown individually into embryos, which were then implanted into pseudo pregnant mother.
Human cloning was attempted by culturing embryos to 8-cell stage under in vitro conditions and for apparent reasons the embryonic cells were frozen for posterity. Who knows that human clones are not produced???
The clonal sheep Meg, Morgan and Tracy were obtained from embryonic cells. Tracy has genetically manipulated to produce sodium channel proteins involved in cystic fibrosis.
Honolulu people in 1997 also obtained a clone by implanting 2n nucleus into an enucleated egg of cumulina mice. Since then many animals have been obtained as clonal animals. In Japan many cows have been developed as clones. Scientists have attempted to produce pig clones without surface antigens belonging to MHC class.
People have claimed that they have cloned transgenic humans and their birth has been heralded all over the world, but no genetic proof for the said claims. Cloning of human beings does not require great technological protocols; in fact with the existing methodologies one can do this in any labs, which have the facility for in human in vitro fertilization and implantation.
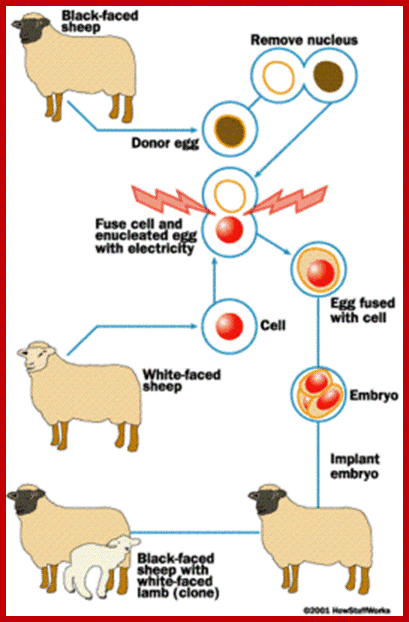

The diagram shows how to develop reconstructed embryo clone;
https://internationalgcsebiology.wikispaces.com/Cloning
Robert A. Godke, Richard S. Denniston and Brett Reggio http://www.lsuagcenter.com/

Transgenic Bull? https://www.pinterest.com/
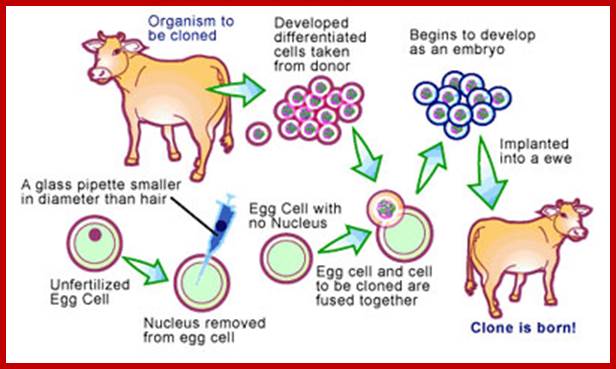
The New Macdonal Pharm; Nuclear Transfer Technology. One method of cloning animals, nuclear transfer involves removing the nucleus from a donor animal and planting it into an egg cell that has had its nucleus previously removed. http://www.scq.ubc.ca/

Brophy.B et al:
;He cloned cows produce high-protein milk; http://www.genomenewsnetwork.org/

Doctorow Found this 'Green Cow from Creative Commons Attribution (2.0) image from nuskyn's photostream; this cow has some human proteins like lysozyme; http://www.dailykos.com/

Scientists clone genetically-modified dog that 'glows' when given antibiotic; ; Cloning of Dog; http://waronyou.com/


Cloned Dog

Fluorescent Cats obtained through Genetic Engineering- green and red- transgenic animals- from South Korea. http://news.softpedia.com/
Macdonald farming-Transgenic Cows.

Animal cloning: Cows; Australian scientists have claimed a world first, cloning a cow using a new technique that produces a healthier embryo. http://www.scq.ubc.ca/

Cloned cows produce high-protein milk; http://www.genomenewsnetwork.org/

Goat n Dog

Stem cells: the costs of not cloning; http://archive.cosmosmagazine.com/
http://www.councilforresponsiblegenetics.org/
Scientists have used cloning technology to transform human skin cells into embryonic stem cells, an experiment that may revive the controversy over human cloning.
The researchers stopped well short of creating a human clone. But they showed, for the first time, that it is possible to create cloned embryonic stem cells that are genetically identical to the person from whom they are derived.
These stem cells could go on to differentiate into heart, nerve, muscle, bone and all the other tissue types that make up a human body; http://www.councilforresponsiblegenetics.org/
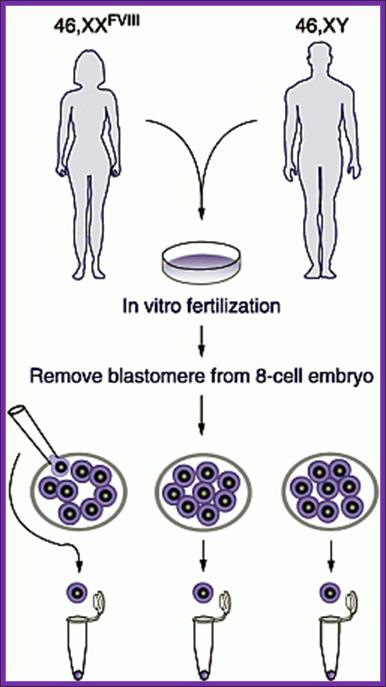
Each blastocysts cells can be implanted in the uterus and grow the embryo.

Idha the mule

Transgenic pig; http://arbg.missouri.edu/

http://www.bloomberg.com/; Kore’s Soom biotech is the world’ Lovely cloned cat; but the first cat cloned was from South Korea.

Calf enginnered; http://butterfly-insect.com/


Cloned piglets, Oh my god! they are so lovely and loving; http://www.answers.com/article

I love these kids; are they cloned children?
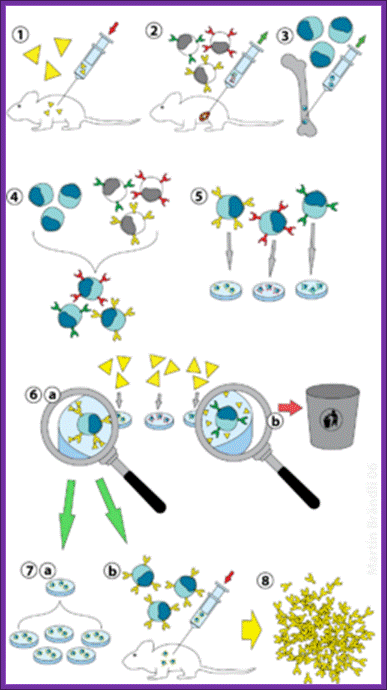
13. Hybridoma Technology:
This ingenious technique was developed around 1975-1976. The production of monoclonal antibodies was invented by Cesar Milstein and Georges J. F. Koehler in 1975. Monoclonal antibodies were once considered as magic bullets that can target a cell, a tissue, molecule and or an infectious agent.
Polyclonal antibodies are produced by B-lymphocytes. When a specific antigen comes in contact with specific receptors of B-lymphocytes, signal transduction induces the activation of specific genes within the lymphocytes and they start producing antibodies against the specific epitope that are presented to the lymphocytes. Epitopes are the antigenic determinants of antigen.
Antigens consists of different motifs, different domains derived from amino acid sequences which generate different secondary structural features. Each of these specific domains are derived from distinct motifs. They are recognized by different B. Cells, for they carry specific receptors for a specific epitopes. If an antigen has twenty different epitopes twenty different species of B-cells get activated and twenty different antibodies are produced in one to one fashion. Production of antibodies to each of the epitopes is not equal, some elicit weak response and some elicit strong response. Thus the antibodies produced are Polyclonal in nature.
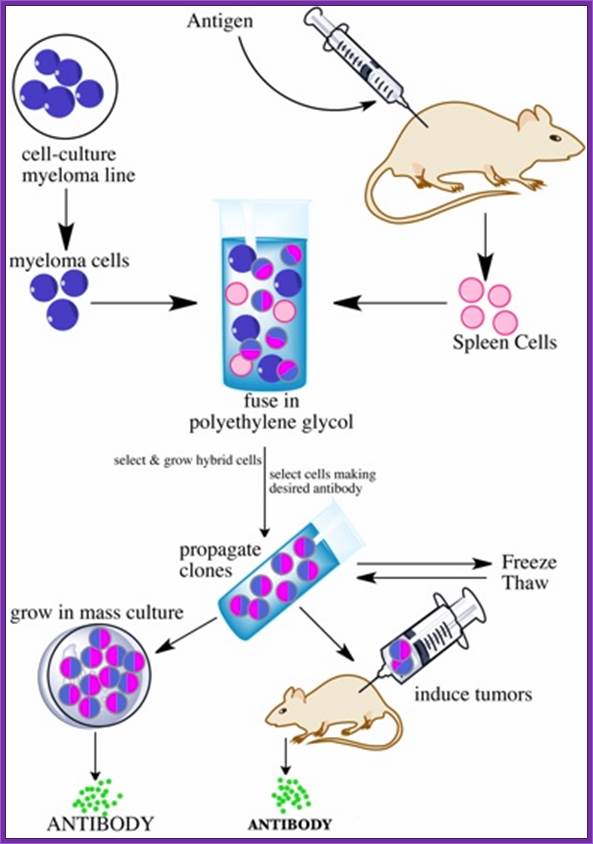
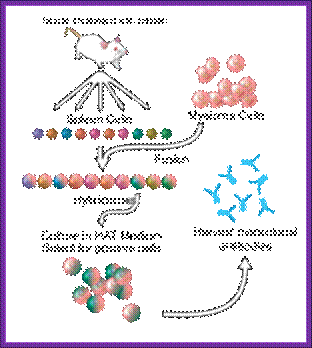
Summary of Hybridoma Method of mAb production; http://php.med.unsw.edu.au/
(1) Immunization of a mouse; (2) Isolation of B cells from the spleen; (3) Cultivation of myeloma cells; (4) Fusion of myeloma and B cells; (5) Separation of cell lines; (6) Screening of suitable cell lines ;(7) in vitro (a) or in vivo (b) multiplication; (8) Harvesting

Many a time’s Polyclonal antibodies are not useful for specific purpose. Yet Polyclonal antibodies are used on large scale for it is easy to obtain such antibodies and cost of production is relatively low.
Monoclonal antibodies are for a specific epitope: Under certain situation one wants to target only one of the epitopes and in such circumstances monoclonal antibodies are very useful; that is why they are called as magic bullets. Production of monoclonal antibodies is possible by Hybridoma technology.
B-Lymphocytes:
B-Lymphocytes are capable of producing antibodies in response to specific antigens.
These cells cannot grow and multiply in an in vitro culture medium. They can survive for some time and use salvage pathway if they are grown in the presence of Hypoxanthine, Aminopterin and Thymidine (HAT) medium. Cells cannot multiply in culture conditions. These cells have HGPRT and Thymidine kinase genes.
They can use hypoxanthine for purine synthesis even though Aminopterin blocks DHFR, they have salvage pathway. The reason is the cells have enzymes called Hypoxanthine Guanosyl Phosphor Ribosyl Transferase (HGPRT).
HGPRT defective Myeloma cells:
They are incapable of producing antibodies. They are the transformed B-cells.
They have the ability to multiply under culture conditions by cell division.
Mutant cell lines, cannot utilize Hypoxanthine and Thymine in the presence of Aminopterin for they lacking in HGPRT and Thymidine kinase (TK).
Protocol: Inject the required antigen subcutaneously to a rabbit skin. After three such injections (second and third are called boosters), sacrifice the animal and take out spleen tissue and isolate B-lymphocytes. These lymphocytes are activated to the injected antigen. The cells are capable of producing Polyclonal antibodies.
B-lymphocytes are then fused to HGPRT (-) Myeloma cells. The hybrid cells now can grow and multiply in culture medium even in the presence of Aminopterin. At the same time they also secrete respective antibodies to specific epitopes. Such cells are called Hybridoma cells.
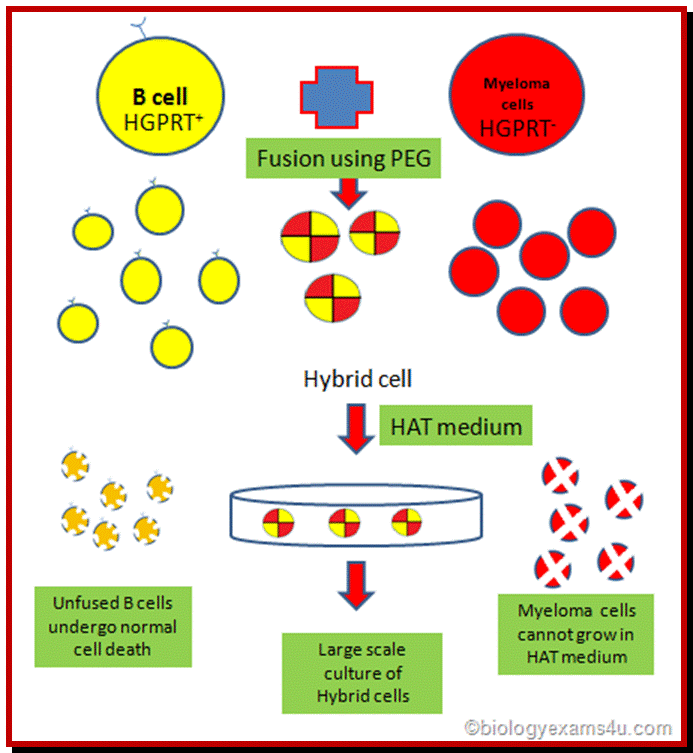
http://www.biologyexams4u.com/
Such cells are diluted and dispensed into multi titer plates in such a way that each well contains one such Hybridoma cell. Such cell lines divide and redivide, grow and produce antibodies to one specific epitope.
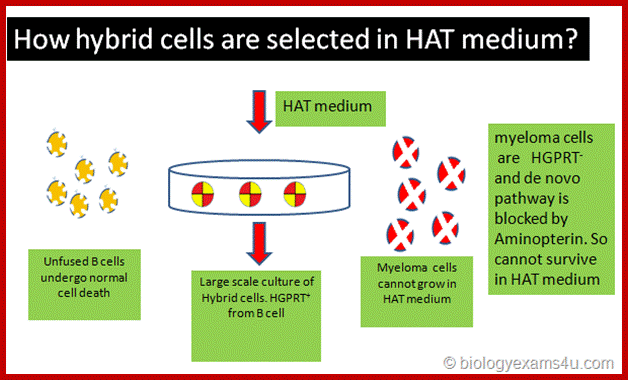
http://www.biologyexams4u.com/
Using a specific epitope from the antigen one can screen cells from each well and pick whatever clone from the titer plate one desires. The same cell line can be propagated and maintained.
Monoclonal antibodies are very useful tools in research.
One can trace the position of an antigen.
Scarce proteins can be purified.
Used for disease diagnosis.
Such cells can be used to treat patients infected with a particular infectious agent.
It is possible to target a specific malignant tumor cell or cells and kill them by directing T-killer cells to them.
21. Molecular diagnostics:
Infectious diseases are generally identified by symptoms, culturing and microscopic examination. And many of the investigations are also done by biochemical analysis. These methods are still in vogue. But certain infectious diseases take a long time to manifest the symptoms and often-early diagnosis leads to miss interpretation. Example malarial infection takes a month or so to fully blown manifestation. On the contrary HIV infection may take a year or so. By the time theses are diagnosed, treatment will be delayed and it can be disastrous. Of late molecular diagnostic tools have been evolved. One such technique is ELISA (Enzyme Linked Immuno Sorbent) Assay) technique has been developed, either using Polyclonal antibodies or monoclonal antibodies. This is certainly very effective, but not all diseases can be identified by this technique and expensive. Yet ELISA is a powerful and highly sensitive diagnostic method, which is used in medicine, research and industry. Definitely a monoclonal antibody technique is very very costly. Along with ELISA protocols, another technique called PCR has been developed to identify not only diagnosis of infectious agents but also to detect any suspected genetic diseases. For example HIV infection can be positively diagnosed by PCR method. Combined with ELISA the diagnosis is 100% efficient.
Antibodies as diagnostic tools:
Antibodies have to be raised for every antigen bearing infectious agent. Most of the antibodies are polyclonal. Some times Polyclonal antibodies cannot distinguish between pathogenic and nonpathogenic germs. Hence people started using monoclonal antibodies, which was found be costly and not within the reach of common folks.
A List of antibodies employed in diagnosis (just few examples):
IgGs for Polypeptide hormones –Few examples:
Chorionic gonadotrophin.
Growth hormones.
Leutinizing hormones.
Follicle stimulating hormone.
Thyroid stimulating hormone.
Prolactin.
Cytokines:
Interleukin 1-8.
Colony stimulating factor.
Tumor markers:
Carcinoma embryonic antigen,
Prostrate specific antigen,
Interlekin 2 receptor,
Epidermal growth factor.
Drug monitoring:
Theophylline,
Gentamycin,
Cyclosporin.
Others:
Thyroxine,
Vit-B12,
Ferritin,
Fibrin degrading factor,
Few examples IgGs against disease causing organisms:
Chlamydia,
Herpes simplex,
Rubella,
Hepatitis-B,
Hepatitis A,
Hepatitis-C,
Schigella,
Mycobacterium,
HIV,
HPTLV,
Cholera toxins,
Botulins,
Polio,
Nematodes,
Malarial antigens,
A large number of viral antigens and bacterial antigens and protozoan surface antigens have been used for developing diagnostic kits. The above list is to give an idea what are few diagnostic IgGs.
In recent years instead of preparing IgGs for every antigen, scientists have used total human lymphocytes and prepared a composite but combinatorial cDNA library for both Heavy chains and light chains. Lambda DNA has been used to prepare such library. This method can generate 10^6 to 10^8 clones, each distinct from the other. Proteins expressed can be screened with diagnostic antigens. Now days the antibodies are generated using genetic manipulation in such a way the antibodies when injected to human, they don’t elicit immune response and such antibodies are called humanized antibodies.
DNA as the diagnostic material:
This has become a sine quo non technology when everything has failed. This technique has undergone lot modification and sophistication. The method is simple and fast. This technique can be used to detect not only disease causing pathogens but also
Genetic defects:
Most important requirement for PCR based diagnostic methods is knowledge of the pathogen’s DNA and its sequence. From the sequence one can generate a set of primers for 5’ and 3’ ends. Known primers can employed to identify. The source of material required is tissue sample, fecal material, urine, blood sample, throat smears or throat wash, a hair follicle. The amount DNA required can be as small as few picograms. In order to combat disease all over the world, WHO has a program to develop 300- 500 diagnostic kits.
Diagnosis of Malarial parasites:
Full-blown disease manifestation takes 15 days to one month. Malarial disease is caused by plasmodium falciparum. Elisa is effectively used, but the parasite often camouflages by withdrawing its surface antigen and projecting another antigen. Here the probe used is highly repetitive DNA sequences. Such sequences when used they are distinguishable from P.falciform with other P.vivax, P.cyanomoglia and other related parasites. Blood sample is more than enough diagnosis of the disease.
Similar highly repetitive sequences have been used to detect disease-causing parasite such as Trypanosome cruzi (chagas disease). Amplification of the said DNA using specific primers it is possible identify this disease with accuracy and other related forms are not detected. It is very specific. This disease has devastated South American population.
Diagnosis of DMD:
Duchene’s muscular dystrophy sex linked disease, generally appears at greater frequency in male children than the female for the females are the carriers. Here the diseased children suffer from muscular wastage for the surface protein Dystrophin is non functional. This protein is found in the myelin sheath at the inner surface covering the axons. The DMD gene is located in the XP21 arm.
The fully processed DMD mRNA is 14 000 nucleotides long. The pre mRNA consists of `60 Exons. The whole gene extends to an area of 20000kbp. The protein has a molecular mass of 600 KD; perhaps it is one of the largest proteins known in eukaryotic organisms. In fact the largest protein is ‘Titin’.
When the DMD gene is cut with Taq-I enzyme, it generates seven fragments. Sites for the Taq-I enzyme is found in few introns. Scientists have developed primers for each of the fragments and the same can be used for PCR amplification. Control DNA generates 7 fragments of the size 10KB, 7.8 kbp, 4kbp, 3.8kbp, 3 kbp, 1.8kbp and 0.6 kbp and they can be discerned on an Agarose gel. If a suspected patient’s DNA is used one will find some segments of the Gene are missing, using multiplex PCR it is possible to diagnose the disease in a short time; and that too with certainty.
The same technique can be employed in identifying phenyl ketoneuria, Sickle cell anemia, Hemophilia, Cystic fibrosis and others.
Diagnosis of Hemophilia:
Hemophilia polymorphism is due to change in restriction sites such as Xba-I and Bcl-I. This is due to mutation in the sequence of these sites. Hemophilia X factor has 26 Exons. Abnormality was found in the 18 Th introns. A pair of primers were developed for amplifying a 142 bp long DNA involving the introns. After PCR amplification of the segment it was cut with Bcl-I and separated on a gel. A patient, whether he or she is heterozygous and homozygous can be discerned from the gel pattern.
|
Control (Homozygous) |
Control (Heterozygous) |
Patient-1 |
Patient-2 |
|
|
|
----- |
------ |
----- |
(-) Bcl-I 142 bp |
|
------ |
----- |
------ |
|
(+) Bcl-I 99bp |
|
------ |
----- |
----- |
|
(+) Bcl-I 43bp |
|
|
|
|
|
|
Multiplex PCR is used to detect mutations in b-Globin genes. Primers are used are fluorescent labeled. Thereby they were able to detect two mutations in b-Globin genes, ands one mutation in alpha Globin gene.
Human Genetic Diseases:
It has been estimated that human genetic disorder and their manifestations in the form of diseases is about 3000 and odd. The diseases can be autosomal or X-linked. Mutations can be dominant, negative dominant, recessive, can be incomplete penetrance, may show variability in expression, show heterogeneity, and or exist in alternate forms of the same gene. Genes are pleotropic.
23. Therapeutical Agents.
Any chemical or biochemical compound designed to combat disease fall into the category called Therapeutical agent. Since the days of Edward Jenner, early 200 years ago, scientists are in hot pursuit of discovering drugs against pathogen. Vaccination against pathogens worldwide is the most effective preventive therapeutical agent next only to antibiotics. Discovery of Antibiotics is a great event in the history of mankind, but over use and abuse of antibiotics resulted in the pathogens have become or becoming resistance to drugs. It is constant and eve going struggle against pathogens and drug discoverers, who dominates that is the question is like survival of the fittest.
Vaccines:
Edward Jenner was an incredible country doctor, using folklore knowledge; he injected liquid from the cowpox pustule into an eight-year-old boy called James Philips. It was a history unsurpassed of any discovery the mankind knows in centuries.
Most of the infectious agents cause infection spread and some of them in epidemic, rarely in pandemic proportions but they can be prevented by vaccination. Any immunogenic proteins injected into human body, elicits immune response against that antigen. The efficiency of response depends upon the epitopes found on the presenting surface of the antigen.
According to ‘WHO’, the number of communicable diseases against vaccine to be developed by 2000 were just 400? But now the target now is much more. Example ‘Dengue” it looks like nobody making efforts to combat this, especially in India. Conventionally vaccines were prepared by using heat killed or formalin killed or inactivated organisms as inoculants. Though maintenance of them and obtain them as pure forms endowed with some difficulties, it is still the cheapest for these have to be prepared in large quantities for global market.
With the advent of Molecular and gene engineering techniques, recent trend is to develop recombinant vaccines and many such products are made available for large-scale vaccination, ex. Vaccination against Hepatitis-B virus. The modern methods have been developed to overcome drawbacks of traditional methods.
Methods employed for developing recombinant vaccines:
- Virulent genes of an infectious agent is deleted or made nonfunctional or dysfunctional and such organisms can be used as live vaccines. It has to be made certain that the organism does not regain that gene or function.
- Live nonpathogenic organism can be used as carriers. These organism can genetically manipulated in such a way an antigenic determinant gene is introduced into the organism where it presents the antigen to immune system, thus such organisms can be used as vaccines. But the antigenic determinant used should elicit strong response.
- Develop alternate host or forms for those organisms which cannot be grown and maintained on large scale, ex. Mycobacterium lepri. These have to be grown and sustained in the sub-epidermal tissues of Armadillo, which itself is rare to find in nature. In such cases the target gene has to be cloned into either bacteria or into yeast cells so large-scale preparation are made easy.
- Some times infectious agents do not kill host cells, instead host immune system attacks infected cells and kills them. It is now possible to create targeted and cell specific killer systems so as to destroy the infected cells.
Subunit Vaccine:
Instead of using the whole virus or bacterial cell or pathogenic animal cells, a specific component of infectious organism is used, such as viral capsid protein, surface glycoprotein, cell wall antigenic protein or glycoprotein can be effectively employed for eliciting strong immuno-response. The said genes can be cloned into expression system and the same can be purified and can be used vaccines. Such vaccines are stable, safe and the antigens are precisely defined and free from extra cellular contaminants. But the process is costly and many a times immune response is good as expected, but that can be overcome by certain modifications.
Subunit vaccine against Herpes simplex virus:
The viruses cause deadly sexual diseases not only in males but also in females, where they act as reservoirs and males as transmitters. Great many people belonging to higher social status have succumbed to these viruses and died with remorse. The virus is an enveloped type and the protein used is protein-D (gD) is a glycoprotein; it elicits good immune response per se. The said protein does not cause any disease or any other side effects.
The gene for this protein has been cloned into transfer vectors or episomal vectors and transfected Chinese Hamster Ovarian cell lines (CHO) produced properly folded and glycosylated proteins which are as good as the typical viral protein. When the gene was cloned in secretory mode the proteins are secreted out of the cell into the media.
Foot and Mouth Disease Virus (FMDV):
This virus has the ability spread across the continents and cause very severe damage to live stock especially Cattles and Swine. This is very severe in South America, even Indian animal population also suffers but Indian medicine has taken care of the problem. The medicine is a concocted extract of Curcumin, Garlic, Oscimum, Gingiber offcianale and Leucas aspera leaves and a little bit fresh capsicum. If this given a week or so the disease is cured. However the world over requirement of vaccine against this virus is not less than more than 2 billion doses.
The surface protein that is very antigenic is the coat protein called VP1. This gene has been cloned but the protein per se was found to be very poor antigenically. The virus possesses ~8000ntds long RNA genome and the cloned was expressed as fused protein with MS2 Replicase as a carrier protein. Even this was not effective in eliciting a good response.
Scientist looked into different motifs of the protein and the same were selected and cloned; such as- from C-terminal 141-160=21aa, 151-150=11, 200-213=13aa, from the N-terminal sequences such as 9-24aa, 17-32aa, 25-41. These subunits were used with a carrier protein and all of them were found to be very immunogenically. However fused segments from141-158 to 200-213 was found to be stimulatory but 1000x weaker than killed viruses. But when the subunit from 142-160 was fused, Hb-surface antigen was found it was highly immunogenic.
Live recombinant Vaccines:
Methods employed- one is use nonpathogenic organism and genetically modify so at express the desired antigenic protein in proper perspective. The second alternative is use pathogenic organism and deletes the virulent gene responsible for causing the disease and retains its antigenic characters.
Live cholera vaccines:
Live vaccines are believed to be more advantageous than subunit vaccines. Cholera is a deadly disease recurs periodically in tropical countries and kills loot of people. Symptoms are fever, abdominal pain, vomiting, diarrhea, which if not take care of will result in death. In many part of the world it is an endemic disease caused by bacteria called Vibrio cholerae, for on infection it colonizes small intestine, which is the target tissue and the bacteria secretes lot of hexameric enterotoxins.
This is the causative agent consists of subunits-A and the other 5 subunits called B. The sub unit-A has ADP-ribosylation activity and stimulates Adenyl cyclase. The A-subunit has A1 domain, which contains functional domain the other part help in joining A to B. They bind to intestinal mucosal receptor and activate intestinal problem.
First deleting the A1 subunit part of the gene by homologous recombination method, in this process tetracycline gene is introduced in the place of A1 subunit segment has developed recombination live vaccine.
Plasmid containing homologous segments and a tetracycline gene id transferred to bacteria Cholera Vibrio. This leads to recombination and deletes the A1 subunit. But this cannot used for it has tetracycline gene. So the enterotoxin gene was isolated and the A1 subunit deleted and plasmid is created containing the deleted enterotoxin gene with homologous segments at both flanking regions. This plasmid is transferred to bacteria with deleted enterotoxin gene. By recombination method the defective enterotoxin gene incorporated and the Tetracycline gene is removed. Such genetically manipulated bacteria are used as live vaccine. It is incredible feet indeed
HB-sAg vaccine:
HB-Ag gene form hepatitis was cloned into a plasmid contain Vaccinia strong promoter. The flanking region of this construct contained homologous segments of Thymidine Kinase gene. When this plasmid and wild Vaccinia viral DNA is co-infected into desired cell lines, by homologous recombination process the Hb-Sag gene is incorporated in the place of Thymidine kinase. This can be screened by Glycover for the absence of Thymidine kinase. This results in the expression surface antigen of Hepatitis, which can be used for vaccination purpose.
Employing the above method a wide variety of genes have been cloned to produce vaccines, ex. Rabies viral G-protein, Sindbis surface antigen, Influenza Np and HA protein, HSV glycoprotein, Vesicular stomatitis N & G protein and many others and used them for vaccination purpose.
Similarly adenoviruses can be used as live viruses. This achieved by deleting replicative function of viruses and desired gene is cloned under the control of early genes. Living viruses is generated using helper viruses. Such viruses have been created for curing cystic fibrosis, a deadly disease among Greek and Cypriot population, where diseased children die before 20 yrs. When recombinant virus id produced with a cystic fibrosis, channel protein gene with flanking regions such as LTR sequences, on delivery the Gene gets integrated into host cell genome. This virus can be delivered into host by nasal spray.
Genetic Immunization:
A cloned gene construct which is capable of producing an antigenically active protein, when biolistically injected into the ears of a mice, the Gene with its borders (LTR) gets integrated and expressed a protein. The protein now acts as an antigen and animal’s immune system responds and mounts antibody production against the protein. This technique has a promising future. When humans are very young as old as 2-3 years it is possible to inject such cloned DNA into body cells to make the child immune to a given antigen. However continuous production of such antigens with in the body will have repercussion effects and how the body can tolerate such continuous stimulation is another question to be answered. Another problem is the quantity of the antigen produced, there is no regulation. I f the production of antigen is made to be regulated with specific stimulation, probably serves the system good.
Use of antibody as drugs:
Coryneybacterium diphtheriae causes a severe disease faster and kill the patient fast. It first shows infected symptoms in the throat and tonsils, where it generates exotoxins, which on circulation can severely damage organs, which are distant from the site of infection.
People use passive immunity against such disease causing agents. By raising antibody in horse against such bacteria and then injecting the serum into humans, results in developing passive immunity. However second time injection with horse serum will be so serious; it may give anaphylactic shock and cause death. Yet the use of antibodies against a variety of disease causing agents and curing the disease is prevalent. New methods and new approaches to the problems have been in vogue. Monoclonal antibodies are once considered as magic bullets, but because of immune reactions to animal antibodies it became imperative develop antibodies, which are human compatible, called, humanized antibodies. Though monoclonal antibodies are expensive, they are still used in diagnostics; tissue imaging and some times they are used as immuno-suppressants.
Among lymphocytes, T cells which differentiate in Thymus cells act as immunological helper cells and also effecter cells. They are involved in rejection tissue grafting. If grafting to be successful T-cell mediated immune response to foreign tissue should be suppressed, thus one can save lives of a large number of human beings. Researches developed antibodies against T cell receptors called CD3. The first monoclonal antibody developed against CD3 in mice is called OKT3. When injected it binds to the receptor and prevents full scale mounting of immune response to tissue grafting. This kind of treatment was first approved by United States food and drug administration. Without the approval no one can try to use trial on human beings at least in the US of A.
Such monoclonal antibodies are still in use in hospitals where bacteremia is endemic and kills lot of patients in USA. Monoclonal antibodies were used to suppress proliferation of breast cancer cells by raising antibodies against specific cell surface receptors on tumor cells. When such antibodies were used the cell surface receptors were blocked and growth of tumor has become stagnant. Further more once the growth of tumor cell is halted, some factor should be added to cause apoptosis of cancer cells only.
Monoclonal antibodies (mAb or moAb) are mono specific antibodies:Targeting mAbs to specific sites:
Blood clotting in vessels leading to brain and heart can lead to stroke and heart attack. The blood clot in vessels is called Thrombus; it consists of a network of fibrin, one of the primary blood-clotting agents. A defect in the endothelial layers of blood vessel is the cause. Thrombus kills millions of people all over the world, especially in developed world.
Plasminogen (86KD), a precursor protein for plasmin, is a serine protease. The precursor Plasminogen is activated by Plasminogen activating factor (70KD). Once activated the plasmin acts on fibrinogen and fibrin and degrades the clot.
Raise antibodies to human fibrin and conjugate it with Plasminogen activating factor in such a way the enzyme’s active site is not disturbed. When such conjugated composite factor is injected, it homes to the site wherever fibrin is present. As most of the clots contain fibrin, the plasmin a serine protease acts and hydrolyses these proteins using serine sites in the protein. Plasminogen is also activated by Urokinase (54KD). Even Streptokinase takes part in dissolving blood clots. It is important to note that using monoclonal antibodies are used to deliver the required enzyme to specific site in the body. This has great implications in curing several diseases. While treating patients additional factors are also added with t-PA to inhibit t-PA activity, other wise it can lead to internal bleeding.
A general representation of the methods used to produce monoclonal antibodies; http://en.wikipedia.org/
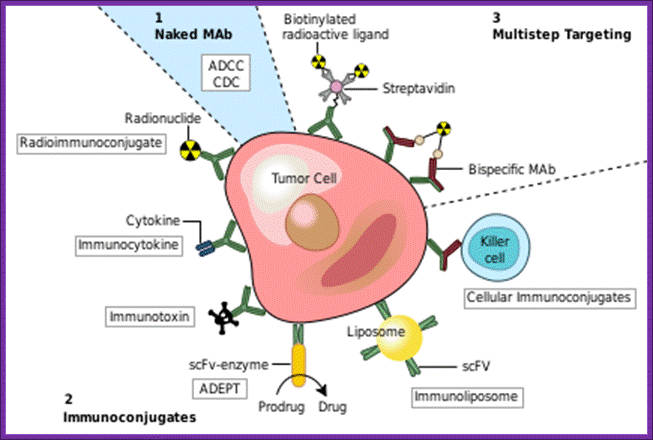
Monoclonal antibodies for cancer. ADEPT, antibody directed enzyme prodrug therapy; ADCC, antibody dependent cell-mediated cytotoxicity; CDC, complement dependent cytotoxicity; MAb, monoclonal antibody; scFv, single-chain Fv fragment (WIKIPEDIA).
The first FDA-approved therapeutic monoclonal antibody was a murine IgG2a CD3 specific transplant rejection drug, OKT3 (also called muromonab), in 1986. This drug found use in solid organ transplant recipients who became steroid resistant.[18] Hundreds of therapies are undergoing clinical trials. Most are concerned with immunological and oncological targets (Wikipedia).
|
Antibody |
Brand name |
Approval date |
Type |
Target |
Indication |
|
ReoPro |
1994 |
chimeric |
inhibition of glycoprotein IIb/IIIa |
||
|
Humira |
2002 |
human |
inhibition of TNF-α signaling |
Several auto-immune disorders |
|
|
Campath |
2001 |
humanized |
|||
|
Simulect |
1998 |
chimeric |
|||
|
Benlysta |
2011 |
human |
|||
|
Avastin |
2004 |
humanized |
Vascular endothelial growth factor (VEGF) |
Colorectal cancer, Age related macular degeneration (off-label) |
|
|
Adcetris |
2011 |
Chimeric |
|||
|
Ilaris |
2009 |
Human |
|||
|
Erbitux |
2004 |
chimeric |
|||
|
Cimzia |
2008 |
humanized |
inhibition of TNF-α signaling |
||
|
Zenapax |
1997 |
humanized |
Transplant rejection |
||
|
Prolia , Xgeva |
2010 |
Human |
|||
|
Soliris |
2007 |
humanized |
Complement system protein C5 |
||
|
Raptiva |
2002 |
humanized |
|||
|
Mylotarg |
2000 |
humanized |
|||
|
Simponi |
2009 |
Human |
Rheumatoid arthritis, Psoriatic arthritis, and Ankylosing spondylitis |
||
|
Zevalin |
2002 |
murine |
Non-Hodgkin lymphoma (with yttrium-90 or indium-111) |
||
|
Remicade |
1998 |
chimeric |
inhibition of TNF-α signaling |
Several autoimmune disorders |
|
|
Yervoy |
2011 |
Human |
|||
|
Orthoclone OKT3 |
1986 |
murine |
Transplant rejection |
||
|
Tysabri |
2006 |
humanized |
alpha-4 (α4) integrin, |
||
|
Arzerra |
2009 |
Human |
|||
|
Xolair |
2004 |
humanized |
immunoglobulin E (IgE) |
||
|
Synagis |
1998 |
humanized |
an epitope of the RSV F protein |
||
|
Vectibix |
2006 |
human |
epidermal growth factor receptor |
||
|
Lucentis |
2006 |
humanized |
Vascular endothelial growth factor A (VEGF-A) |
||
|
Rituxan, Mabthera |
1997 |
chimeric |
|||
|
Actemra and RoActemra |
2010 |
Humanised |
|||
|
Bexxar |
2003 |
murine |
Non-Hodgkin lymphoma |
||
|
Herceptin |
1998 |
humanized |
|||
Recently, the bispecific antibodies, a novel class of therapeutic antibodies, have yielded promising results in clinical trials. In April 2009, the bispecific antibody catumaxomab was approved in the European Union.
Abzymes:
By genetic manipulations it is possible to convert antigen-binding site into an enzyme active site. This product has the ability to recognize the specific substrate as well as to perform enzyme activity.
Use of mABs in treatment has created problems for they themselves act as antigens, so they have modified the IgG in such way they act and behave like human antibodies (humanized). In most of the cases monoclonal antibodies were obtained from mice, and mice antibodies are not human antibodies and they develop resistance to the use of antibodies as therapeutic agents. So scientist started to look for human cell lines to generate good amount specific kind of IgGs against a specific antigen.
Developing Humanized IgGs (HIgGs):
Several strategies were employed. First they isolated human B-lymphocytes by tagging fluorescent antigen and then they are separated by flow cytometry and got reasonably enriched population of specific B-lymphocyte. Then human antibody producing lymphocytes are fused with human myeloma cells. Unfortunately the hybrid cell lines were found to be unstable. Instead they transformed lymphocytes with Epstein Barr viruses. Such transformed cells were viable, they divided and secreted IgGs, but alas! The yield was very very poor.
The other strategy is to clone a human IgG gene, both L and H chains, and transform embryonic cells of the mouse and obtain the transgenic mouse and stimulate mouse for specific antigen and get specific antibodies.
In another method they obtained specific antibody gene for a specific antigen. By genetic manipulation it is possible to replace human Fc fragment in the place of mice Fc fragment. Similarly even the antigen binding hyper variable region can also be replaced. Such genes were transfected to known mammalian cell lines and expressed IgG were found to be human compatible. But it is yet to be realized to produce such Abs on large scale.
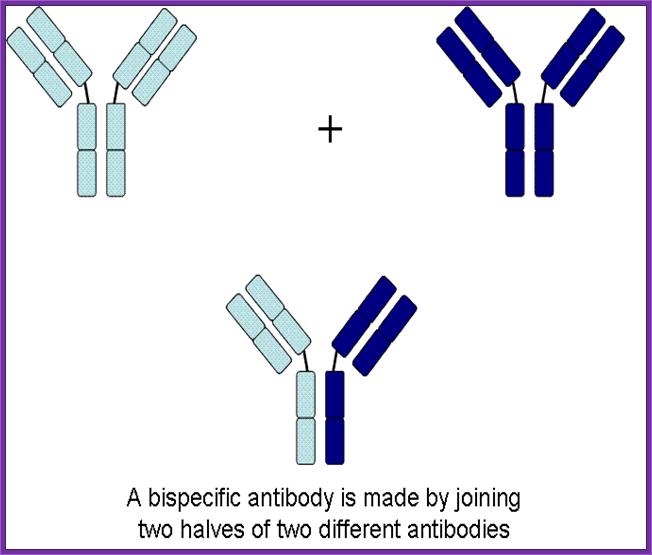
Use of Bispecific Antibodies:
Produce two different but specific antibodies; say one for tumor cell surface receptor and the other for specific T-cell receptor. When such antibodies were denatured and mixed to renature appropriately, one obtains antibodies containing two specificities called Bispecific antibodies.
Another way to generate Bispecific antibodies is to genetically manipulate hyper variable sites to the designed purpose. When such Bispecific antibodies are employed one can target two systems simultaneously. Bispecific antibodies, say one for specific T- killer cells and another for tumor cell, T-killer cells can be targeted to tumor cells.
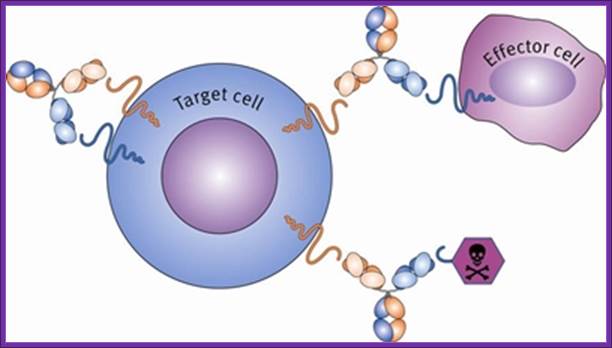
In the case of malignant Hodgkin’s melanoma there are a large number of tumors infiltrating T-cells. And tumor cells have their own specific antigenic receptors. Raise antibodies separately for these two types of cells. Generate hybrid IgGs. Collect TIls (Tumor Infiltrating Lymphocytes) as much possible from the tumor and treat with specific Interleukin to activate the T-cells into killer cells. Inject T-killer cells and Bispecific antibodies. They bind to T-cell and bring them to tumor cells and bind. The activated Tills destroy tumors and clinically found such tumors were regressed.
Micromet - Biting Cancer (Part I):
http://seekingalpha.com/
In the context of cancer therapy, Bispecific antibodies are usually designed to redirect immune cells to tumor cells, in a way that will lead to the attack of the tumor. One arm is directed against a target on a cancer cell while the other arm is directed against a target on the immune cell. In most cases, bispecific antibodies must not only bring the immune cell closer to cancer cells but also activate it to attack the tumor. In order to achieve both of these tasks, there is a need to identify specific targets on immune cells that can be activated by antibody binding, or find supplementary ways to achieve this activation. Naturally, there is a large number of options when designing a bsAb. One variable is the target on the cancer cells, another variable is the type of immune cell to be recruited, while a third variable is which structural element (antigen) on that specific immune cell should be targeted. This is much more complicated than designing mono-specific antibodies, in which only the target on the cancer cells should be chosen.
Genemab- Next Generation Technology
Genmab is creating knowledge and intellectual property around Fab-arm exchange and the generation of bispecific antibodies1-3, thereby establishing a strong basis for the DuoBody platform.
; Cytotoxicity CDC Complement dependent toxicityMab monoclonal;Gene Mabs; www.suggestkeyword.com
Uni-BodyTechnology:
UniBody is a
proprietary antibody technology that creates a stable, smaller antibody format
with an anticipated longer therapeutic window than current small antibody
formats, based on pre-clinical studies to date. A UniBody molecule is about
half the size of a regular type of inert antibody called IgG4 and binds with
only one antibody arm to a therapeutic target. UniBody molecules are expected
to be cleared from the body at a lower rate than other antibody fragments based
on the preclinical studies to date. Unlike other antibodies which primarily
work by killing targeted cells, a UniBody molecule will only inhibit or silence
cells, which could be an advantage in the treatment of diseases such as asthma
or allergies.
New antibody delivers a double blow ; 10:15 13 December 2003 by Andy Coghlan
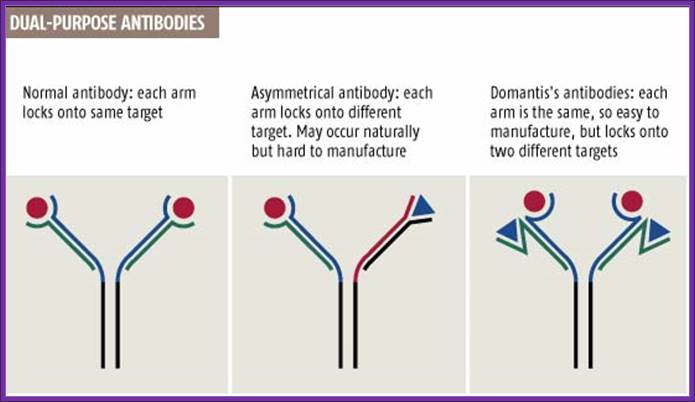
http://www.newscientist.com/
Normal antibodies have two identical arms, each made of two chains, that both bind to the same target (see graphic). But in 1999 Rob Aalberse, of the University of Amsterdam, reported that the body also produces dual-target antibodies, in which each arm locks onto a different target (New Scientist print edition, 18 September 1999).
Cancer cells, for instance, could be better targeted by antibodies that recognize two distinguishing features instead of just one. "With two targets, you'd enhance specificity, so sparing more healthy cells," says Tomlinson, who unveiled the method last week at an antibody conference in San Diego, California.
Epigenetically inducement of vigor in older people:
Transfusion of younger blood or cells into older people has been subjected to studies, but it works in Mice. This provides invigoration of older tissues in older people by younger cells derived from young people. I can request my grandson to provide such cells to me for transfusion.