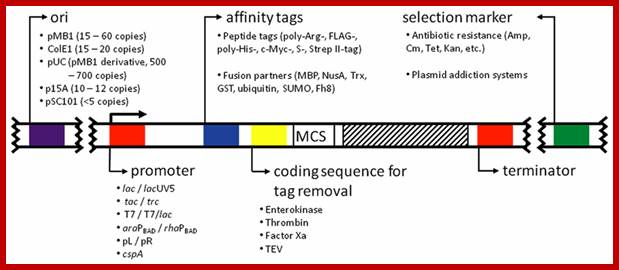
Molecular Tools:
Expression Vectors:
Prokaryotic:
Characters of expression vector depend upon the kind of biosystems one uses and what you want from such structure. For prokaryote one requires one kind of vectors and for eukaryotes one may require different vectors depending upon whether it is a mammalian cell, insect cell line or plant tissues.
· Whatever may be the system, the basic requirement for any expression system is a promoter cloning site(s) next to it and transcriptional terminator. The promoter is the region, which is recognized by the RNA polymerase and initiates transcription and transcribes any on its way. As the transcription progresses it has to have region where the transcription has to be terminated, if the cloned material has such sequences, it is OK other wise one has to provide such sequences for transcriptional termination.
· In addition, the vector should have an origin suitable for replication initiation in a particular bacterium or any host and one desires to have an origin which produces high copy numbers. If the vector requires an origin for another system one has to provide suitable origin of eukaryotes also. Then such vectors become shuttle vectors.
· The vector has to have a selection marker gene such as antibiotic resistance gene suitable for the host.
A gene with its promoter, operator, coding and terminator regions- structural features of a Gene; http://www.frontiersin.org/
· Another most desirable feature of the vector is to have regulator elements so that one makes the gene to express when one needs.
· In addition, one may have to use certain sequences between the promoter elements and the insert, which on transcription provide sequences for translation that may help targeting the protein to certain destination.
· The most important aspect of cloning into a vector is to align the insert DNA in such a way that once the promoter is used for initiating transcription, the transcript should possess a proper leader sequence containing Shine Delgarno for bacteria and Kozak sequence for eukaryotic systems for initiation of translation. The ultimate aim for cloning is to get an expressed product from the cloned DNA material.
· One has to choose a promoter for expression for a particular tissue. It should have surrounding elements which respond to certain stimulus or may act as enhancer sequences which help is the efficiency transcription.
I. Prokaryotic Expression Vectors:
pLac-Z expression vectors:
· Lac –Z promoter operator is in frame with lac-Z alpha fragment (the NH3 terminal part of Galactosidase gene. Multiple cloning sites are found in the border of NH3 end including ATG sequence. The presence of such restriction site sequences should not disturb the functional activity of the protein, which complements with the omega fragment of the Lac-Z produced by the bacterial cell as the complement. If any gene is placed in proper frame in the MCS the protein expressed will be is fused form. The expression of the gene can be regulated by IPTG (Isopropyl thio b-Galactoside).
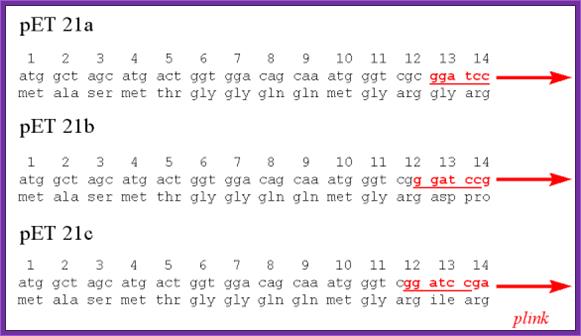
pET Expression Vector:
· The size of the vector is 5700bp
· It has T7 promoter adjacent to lac operator.
· Next to it is a sequence called Shine Delgarno sequence.
· Adjacent to S/D sequence there are few cloning sites such as Nde I, Nhe I and BamH I, at the end of which is T7 phage transcriptional terminator is found.
· For the expression of this gene the bacterial cell should provide T7 RNA polymerase, which is under the control of Lac-Z operator/promoter.
· The repressor produced by the bacterial lac-IQ can be regulated by IPTG.
---T7 P—Lac-O—S/D—(sequence tags)-Nde I-Nhe I-BamH I----T/t--,
(T/T= Transcriptional terminator)
---T7 P—Lac-O—S/D—(sequence tags)-Nde I-Xho I-BamH I----T/t-,
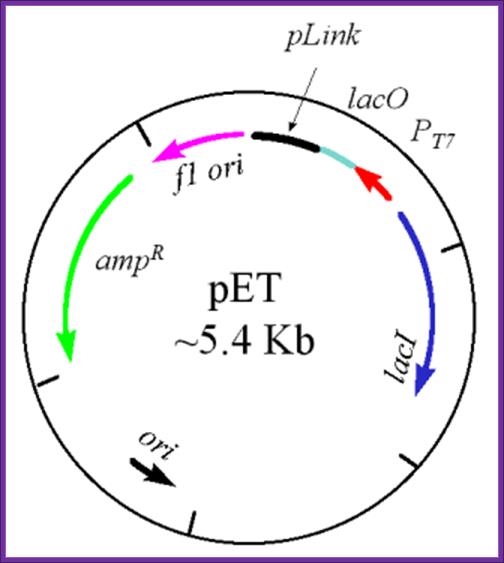
The pET Vector. This plasmid contains a drug resistant marker for ampicillin resistance (green), the lacI gene (blue), the T7 transcription promoter (red), the lac operator region (pale green) 3' to the T7 promoter, and a polylinker region (black). Also, there are two origins of replication - one is the f1 origin which enables the production of a single stranded vector under appropriate conditions, and the other is the conventional origin of replication. This image was used with permission from Dr. Michael Blaber, of Florida State University. pET expression Vector: http://www.bio.davidson.edu/
http://www.mikeblaber.org/
pTac expression Vector:
· This has the combination of Trp and Lac-Z promoter –operator elements, which is inducible. It is considered to be a very efficient promoter.

---T7 P—Lac-O—S/D—Nde I-Nhe I-BamH I---T/t--,
---T7 P—Lac-O—S/D—Nco I-Xho I-BamH I—T/t---,
---T7 P—Lac-O—S/D—I-BamH I-Sma I-R1—T/t--,
· Cloning into all the three cloning sites at least in one gets the clone in proper reading frame.
tac Vectors for High Level Bacterial Expression
· Options for cytoplasmic expression or secretion
- FLAG® vectors for highly sensitive detection and purification using ANTI-FLAG® antibodies, resins, and plates
· MAT™ vectors for high quality purification using HIS-Select™ resins and 96-well plates
· N- or C- terminal fusions
· SHIFT™ vectors for expression in all three reading frames
· Enterokinase removal of N-terminal FLAG tags
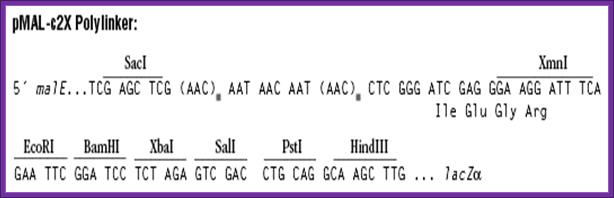
Mal E expression vector: A fusion protein product:
· The size of the vector is 6700bp.
· It has lacIQ-R.
· It has Amp^+ gene.
· It has pTac promoter-operator.
· Maltose binding protein sequence is added next to the promoter for in expression mode.
· A lac-Z alpha part is added to the maltose binding protein as a fusion protein. Maltose binding protein is 42 KD.
· A multiple cloning site is inserted in the N- terminal part of the lac-z alpha segment, so any gene introduced in this site produces white colonies, at the same time it generates fusion protein.
· Such proteins can be purified on maltose columns for the columns are loaded with certain factors, which can cleave the protein at required position.
· Clones can be identified by immunoprecipitation against Matose binding protein or His tag
General notes:
A gene or open reading frame is inserted into a restriction site of the vector polylinker, in the same translational reading frame as the malE gene (encoding maltose-binding protein). Insertion of the DNA fragment interrupts the malE-lacZa fusion pre-existing on the vector, affording a screen for inserts on the proper indicator plates. The fusion protein thus produced can be purified by amylose affinity chromatography. The sequence coding for the four amino acids Ile-Glu-Gly-Arg is present just upstream of the XmnI site. This allows the protein of interest to be cleaved from maltose-binding protein with the specific protease Factor Xa. Fragments inserted in the XmnI site (cleaves GAAGG↓ATTTC) will produce a fusion protein that, after Factor Xa cleavage, contains no vector-derived residues on the protein of interest.
http://66.155.211.155/nebecomm
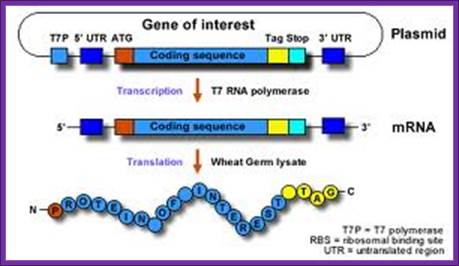
coupled= reaction takes place in one tube without intermediate handling steps; https://www.5prime.com
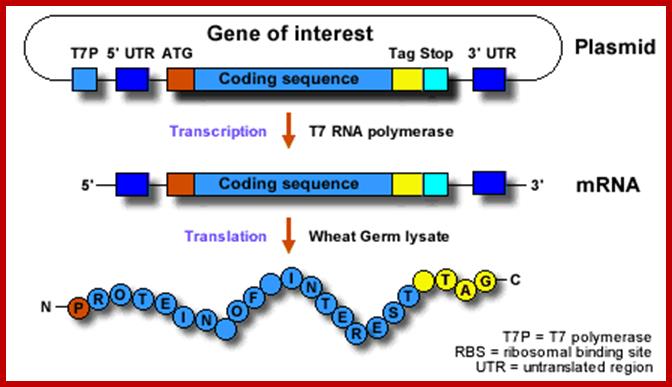
combined = taking place in one reaction tube without intermediate handling steps (e.g., mRNA isolation), but separated in time due to different [Mg2+] optima; To use the system, the gene of interest must be positioned:
· Downstream of a T7 promoter and an in-frame start codon for translation (please note that in the case of eukaryotic transcription there is no defined terminator region like in E. coli)
· Between an optimized 5´- and 3´ UTR (untranslated region)
This can be accomplished by using PCR to incorporate the relevant upstream and downstream sequences (RTS Wheat Germ Linear Template Generation Set, His6-tag) or by using standard cloning methods to insert a gene of interest into an expression vector (RTS Wheat Germ pIVEX His6-tag Vector Set).
Coding sequences for an affinity tag are generally included either in the pIVEX expression vector or the PCR primers of the RTS Linear Template Generation Set to facilitate purification and/or detection after expression.www.5prime.com
IMPORTANT to NOTE:
Unlike in E. coli lysates, in vitro transcription and translation in eukaryotic lysates (e.g. wheat germ) have very different [Mg2+] optima. Highly efficient protein synthesis therefore normally requires the separate preparation of mRNA (high [Mg2+]) prior to translation (low [Mg2+]). In contrast to this, performing reactions under CECF conditions with RTS Wheat Germ kits allows to go from high to low [Mg2+] in the course of the same reaction. As a consequence, both steps can take place with much higher efficiencies. https://www.5prime.com
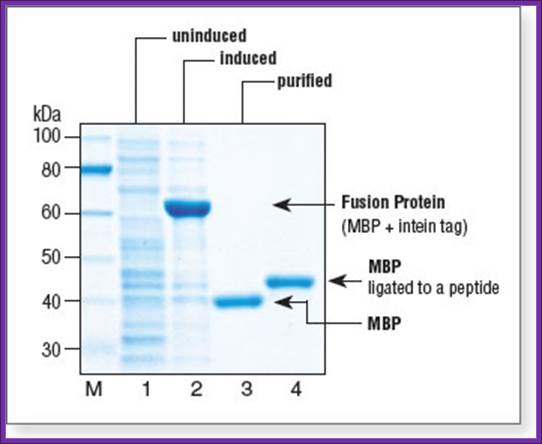
Lane 1: uninduced cell extract. Lane 2: induced cell extract showing expressed fusion protein. Lane 3: MBP fractions eluted after inducing cleavage overnight at 4°C. Lane 4: MBP ligated to purified Maltose binding protein
https://www.neb.com/products/
Fusion protein products: Histidine Tag:
· It is possible to introduce certain sequences in frame so the cloned gene product is produced as fusion proteins. The N-terminal part of the expressed protein is used for purification. Such tags can be Histidine tags the fused protein can be purified using Nickel column. A sequence such as NH3-L-V-P-R-G-S * (MCE), the fusion protein is cleaved with Thrombin which cuts the protein at serine (*). Similarly, one can introduce sequences such as NH3-I-E-G-R*-(MCE), which can be cut with Factor Xa, the position of cutting is marked by (R*). Introduce desired DNA in th reading frame with in the cloning sites
· Inserting the coding segment of the DNA into these will generate a proper recombinant DNA for transcription.
One can generate fusion products to be purified by adding sequences before the actual start codon of the desired gene. The product can be targeted to be secreted into periplasmic space or to be targeted into cell inclusions or the protein can be purified by column chromatography or by cutting the tags by using certain specific proteases or chemicals.
· While cloning one can use expression cassettes for the desired purpose.
I=P=I—S/D—I—ATG-signal sequences-ATG----X gene-----T/t
I=P=I----S/D—ATG-His tag---ATG—X gene---------------------T/t.
I=P=I---S/D—ATG-I-E-G-R*-ATG---X gene-----------------------T/t,
I=P=I—S/D---ATG-L-V-P-R*-G-S--ATG—gene—---------------T/t-,
Expression and Purification of Glutathione-S-Transferase Fusion Proteins
- Donald B. Smith1,
- Lynn M. Corcoran2
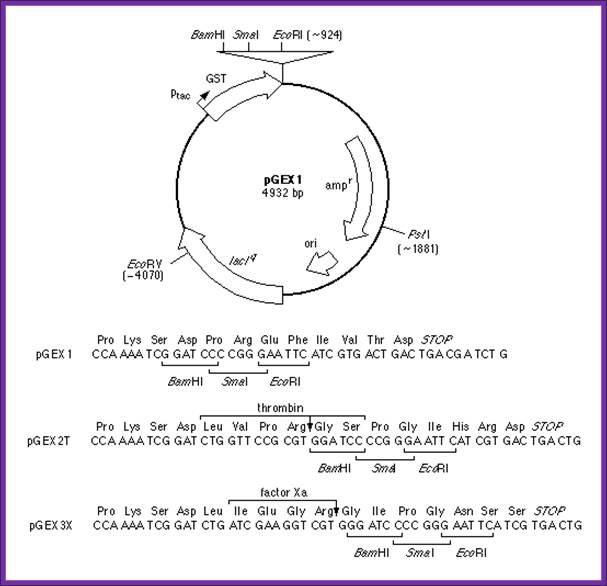
Current protocols in Molecular Biology; http://geguchadze.com/
- This unit describes how pGEX vectors can be used in bacterial systems to express foreign polypeptides as fusions with glutathione-S-transferase (GST). In general, such fusion proteins are soluble and are easily purified from lysed cells under non-denaturing conditions by absorption with glutathione-agarose beads, followed by elution in the presence of free glutathione. Potential applications of the pGEX vectors include the expression and purification of individual polypeptides (including short peptides) for use as immunogens and as biochemical and biological reagents, and in the construction of cDNA expression libraries. This protocol describes production and screening of pGEX transformants and purification of milligram quantities of fusion proteins from 1-liter cultures. The commentary describes several modifications to the expression and purification protocol that may be useful in cases where fusion proteins are insoluble or unstable.
Some of the sequences that generate fusion proteins can be cut with specific reagents or enzymes to release the gene product.
------D*P----, cut with acid (acid pH, 70%formic acid 48hrs).
------N*G----, cut with hydroxylamine pH 9, at 45^oc 4hrs.
------R*--- or ---K*----, cut with Trypsin pH 7-9.
------R*-----, cut with Clostripain pH8.
------N-N-N-N-K*, ---cut with Enterokinase.
------G-P*---, cut with collogenase.
------F-A-H-Y*--, - cut with H64A Subtisilin pH 8.
------L-V-P-R-*-G-S, cut with thrombin.
------R*-X—, endo Proteinase.
------G*-X, cut with endopeptidase (V8 protease).
------E-*A—, can be cut with STE diaminopeptidase.
------Y*-K*-R—, KEX 1 protéase.
Secretary signals to bacterial periplasmic space:
M-K-Q-S-T-I-A-L-A-L-L-P-L-L-F-T-P-V-T-K-A-*-R—
II. Eukaryotic expression vectors:
The design of eukaryotic vectors depends upon the type of system or tissue used and what gene to be expressed and in what way.
In most of the cases the eukaryotic expression vectors designed for manipulation in the bacteria to begin with, it is only then they are transferred to eukaryotes. So the vectors are called shuttle vectors.
Somme of the vectors are designed to be integrated into the host chromosomal DNA and some are designed for transient episomal expression.
· The desirable size of the vector should be as small as possible.
· It should contain replicative origin for bacterial system as well as eukaryotic system one chooses.
· The vector should contain specific selection marker gene for screening in prokaryotic and eukaryotic systems.
· Vector should contain a strong promoter, which can be a general promoter to be expressed in all types of cells or the promoter should be tissue specific type to be expressed only in kind of tissue.
· Another desirable feature is in having regulatory or regulatable promoter (like inducible or repressible), such as having response elements, activator sequences or enhancer sequences or the combination of them.
· For screening and selecting transformed cells a desirable marker gene inclusion in the vectors is almost essential.
· Cloning site or cloning sites next to the promoter elements has to be designed in such a way it should generate a proper leader sequence at the beginning of the transcript perhaps with Kozak elements and there should be a transcriptional terminator sequence such as pol (A) signal block.
· Depending upon the needs one can introduces sequences for targeting the gene product to specific destinations.
· In another novel type of situation, it is possible to create an artificial chromosome similar to that of YAC, where desired genes are introduced under the control of regulatory elements for expression.
III. Yeast expression vectors:
· Yeast is a Eukaryotic system.
· It is a unicellular organism.
· One can obtain mutants such as nutrient mutants or and conditional mutants.
· Mutant can be maintained.
· They can be grown into large numbers and density.
· Like other eukaryotic systems the proteins fold properly.
· The system provides glycosylation of proteins, but it may not be similar to mammalian system.
· The introduced gene product may be made to secrete it products into medium.
· Transformation and culturing of the cells is relatively easy and cost effective.
Yeast Integrative plasmids (YIP):
· The size of the vector is 5.5kbp,
· It has an Ori site for replication and a selection marker gene (Ampicillin gene) for E.coli cells.
· Plasmids are unable to multiply in yeast cells.
· The plasmid has URA3 selection marker gene for integration into the host nuclear DNA by homologous recombination. A defective gene of the host is replaced by functional gene. Similarly, one can knock out a functional gene.
· One can insert a gene in expression mode with border sequence which are homologous to certain hast DNA sequences. There are few Restriction sites such as Puv II, Cla I and HinD3 into which such expression cassettes with homologous border sequences and a desired gene can be introduced.
===Homologous DNA I--P------X gene----I-homologous DNA===
· The border sequences help in integration of the cloned gene into host chromosomal DNA.
· If the border sequence of a host gene and new gene is introduced in between them, by homologous recombination a host cell gene removed and, in its place, a foreign gene gets inserted. This leads to gene knock out
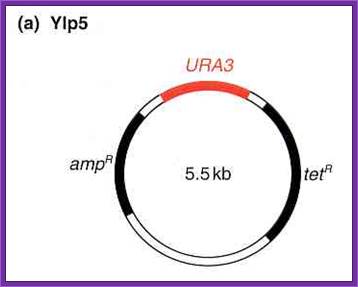
Such gene knockouts will enable researchers to understand the effect of single gene introduced into an organism ~---Ori—Amp^+--R1—Cla I—D3---URA3—Sma I/Xma I--~~
~~ = Ends of the plasmid which is in circular mode.
URA3 acts as the homologous segment.
· Once the gene is integrated by amplifying the clonal cells, one can go on and on in producing the required product.
Yeast Episomal expression vector:
· The plasmid is circular mode.
· The plasmid size is 7.769 kbp.
· It has ColE 1 origin for bacterial manipulation.
· It has bacterial selection marker gene.
· It has URA3 as a selection marker gene for yeast cell. It has 2u plasmid DNA segment with STB chunk (2000bp long) for replication in yeast cells. This helps for the plasmid to replicate in the nucleus and produce a copy number of 100-200 per cell.
· This high copy number will produce greater yield of the gene product.
· It also contain few cloning sites such as Sma I / Xma I, Bpu I, Cla I and Aat II.
· Into these sites one can clone a gene in expression mode using a expression cassette.
· Yeast autonomously replicating plasmids’ ARS sequence component is present, while in yeast episomal plasmid a large component of 2u DNA is present. But adding CEN sequences it can stabilize YRS plasmid, which is called “YCP”.
Yep13

http://biotechhelpline16.blogspot.in/
· Yeast autonomously replicating plasmid produces moderate copy numbers, while yeast episomal plasmid produces high copy numbers.
~Tet^+---Sma I / Xma I—URA3—Bpu I- Cla I----2u DNA- AaT II-Amp^+
Expression Cassettes:
For direct expression:
Promoter /Regulator—G/ACC ATG.G-----------X gene--- T/t.
For secretion:
Promoter /regulator—AAA ATG Sec. sequence--A/GCC ATG.G---X-gene—T /t.
Ex:P-GAPD—ATG---X gene---poly (A)---.
P-GAPD = Phospho Glyceraldehydes promoter.
Using such plasmid with expression mode Zn / Cu Super Oxide Dismutase (SOD) gene has been cloned under the promoter of PGALD. This expression vector in yeast produced a genuine protein used on patients with cross-reactions. Such plasmids have been used in the production Hirudin an anticoagulant produced by leaches called Hirudo medicinalis. When Hirudin is used as anticoagulants they did not elicit any immune responses. Both these products are used during operation.
During blood transfusion ionic radicals are produced. They are very dangerous for they react with other cellular enzymes and compounds. But Super Oxide Dismutase reacts with super oxides and converts them into H2O2, which is then cleaved into H2O and O2 by another enzyme called Catalase. The SOD can also be used against inflammation disease such as Osteoarthritis, Rheumatoid arthritis, Scleroderma and similar disease.
Though the yield of required proteins is good, in certain cases the glycosylation is not same as that of human products; so one has to resort to cell lines, which produce products similar to human proteins.
Yeast expression vectors are used for producing recombinant products such as SOD (Super oxide Dismutase), Bovine Lysozyme, Hepatitis-B surface antigen, Malaria circumsporozoite protein, HIV-1 envelope protein, Hepatitis-C viral protein, Human EGF, Insulin like growth factor, Proinsulin-Insulin, Platelet derived growth factor, Fibroblast growth factor, Alpha 1 antitrypsin, Blood coagulating factor XIIIa, Hirudin and others
Some of the yeast expression vectors used contains specific promoters, which are very efficient, and they can also be regulated.
For example an expression vector for integration was developed for another yeast related fungus called Pichia pastoris. This vector has a promoter derived from Alcohol oxidase or ethanol oxidase, which is inducible. Next to the promoter it has cloning site and transcription terminator sequences. The vector also has Aox 5’ and Aox 3’ sequences for integration and a functional gene His for selection. For transformation of Pichia pastoris- yeast cells, the vector is linearized. These cells are highly productive and respond very well for induction with alcohol. Using such systems Hepatitis B surface antigens were produced on large scale.
A list of yeast promoters:
The basic construction of the vector is-
---Promoter-----X-gene—Ttr--
|
Promoter |
Full name |
Fold of induction
|
|
PGK |
Phospho Glycerate kinase |
20 fold |
|
ADH1 |
Alcohol dehydrogenase, induced by ethanol and glycerol, suppressed by glucose |
20 fold |
|
GAP |
Glyceraldehyde 3-P dehydrogenase |
20 fold |
|
GAL1 |
Galactose utilizing |
1000 fold |
|
GAL10 |
Galactose utilizing |
|
|
ADH2 |
Alcohol dehydrogenase, repressed by glucose |
100 |
|
PHO5 |
Acid Phosphotase |
200 |
|
MFalpha1 |
Mating factor a 1 |
105 fold if the TM is shifted to24oC |
|
AOX1 |
Alcohol oxidase |
|
|
ADH/GRE |
ADH growth hormone response element |
|
|
ADH/ERE |
ADH-estrogen hormone response element |
|
|
PGK/ alpha2 |
|
100fold by shifting the temperature |
|
GAP/GAL |
|
150-200 induced by Galactose |
|
CYC1/GRE |
|
50-100 by deoxycorticosterols |
PGK: I-----P-----I-mcsI---//--T/t.
ADH1: I------P-----I-mcsI--//---T/t.
GAP: I------P-----I-mcsI---//T/t.
GAL1: IUASI--I---p---I-mcsI---//T/t.
AOX1:I------P—I-mcsI----//-T/t (Pitchia pastoris)
http://www.cambia.org/
Intracellular expression:
Eliminate all UTR sequences upstream of ATG.
Precede initiator codon with AAAAAATG.
The gene cloned should be free from introns.
Transcriptional terminator region should be GC rich.
For secretion purpose:
Use yeast signal peptide sequences at the 5’ end of ATG.
MFalpha2- by kex2 protease- lys-Arg*--
STE diamino peptidase--*Glu-*-Ala-
Kex1- -*Lys-*Arg-
6. Vectors for animal cells:
Human Papova viral based Vectors:
· They are often called BKV vectors. BKV viruses are icosahedral, 50nm thick and ~5000bp long.
· The viral vectors can be maintained in human cell cultures as extra chromosomal elements with high copy numbers.
· The vectors developed are shuttle vectors.
· The vector contains a large fragment of viral origin and some segment of DNA, which provides elements for high copy numbers.
~~---Dre-MMtr-P—gene-X-Ttr—SV40-P—Kan+ gene- Ttr—BKV-Ori--~~
· Circular module.
· A promoter from Mouse mammary tumor virus has been included.
· The promoter is abutted by a dioxin response element (DRE). Dioxin is orange-G (2,3,7,8 Tetra Chloro di Benzo p-Dioxin (TCDD), it is a toxic substance used by USA army in Vietnam during 1970-73.
· Next to it is cloning site(s) and a transcriptional terminator signal sequence.
· Kanamycin gene resistant gene is placed under Sv40 early promoter.
· It also contains Ori-E and Ampicillin resistant gene.
· When this vector containing a gene is transferred to a human cells, the vector enters into the nucleus and copies into high copy numbers, the number can as high as 2000-8800 per cell.
· As the number increases to the maximum the expression of the gene is induced by adding Dioxin.
· The target for the protein can be designed by using specific signal sequences before the inserted gene in reading frame.
BKV derived composite Shuttle Vector for immunoglobulin subunits:
This kind of vector was used for the production of light chain and heavy chain of a specific antibody. The IgG produced were non-reactive when delivered into human system.
1 st vector (pL):
It has ColE-1 origin.
It has AMP+ gene.
It has promoter derived from b-Actin.
It has T/tr region.
The Light chain gene has been inserted in proper reading frame.
A gene for DHFR has been put in place with a promoter derived fromSV40.
It also contained BKV origin with high copy number element.
2nd Vector (pH):
It contained ColE 1 origin and an Amp + gene.
It has promoter from b-Actin gene where the IgG’s heavy chain has been cloned with T/tr region at its end.
Kanamycin plus gene has been placed under MM tr promoter.
Along with BKV origin and high copy number element has been incorporated.
When both these constructions are transfected and selected on Methotrexate and Kanamycin. In fact in the presence of Methotrexate the DHFR gene by unknown mechanism amplifies to high numbers.
Using this technique researchers raised IgGs against the receptors found on malignant lymphoma cells, also called Hodgkin lymphoma. However the IgG genes were so modified, when they were introduced into human body without any immune response against IgG, so they are called “Humanized antibodies”. In this case IgGs raised are against malignant lymphomas cellular surface glycoprotein. The surface antigens actually facilitate and activate cell for further proliferation by cell-to-cell contact. Blocking such lymphomas by IgGs prevents the further proliferation and surprisingly the malignant cells regressed.
Similarly one can use Glutamine synthase as selection marker gene, which is resistant to Methionine sulfoximine (MSX). In this case the cell need lack this gene.
SV40 derived vectors:
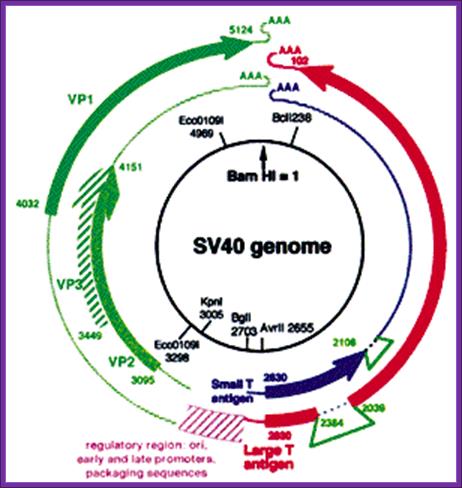
SV 40 has late promoters and early gene promoters; they can be used for generating a functional vector, which can be propagated in monkey or human cell lines. The virus is isometric and it non-enveloped virus, It is made up of three types of capsid proteins and all are derived from the same transcript by alternate splicing. The DNA is double stranded, and circular, 5243 bp long. The virus infects through cellular receptors, and the viruses are taken in by endocytosis. Inside the cytoplasm the DNA is delivered into the nucleus. As the ds viral DNA enters the nucleus it gets associated with host histones and becomes a mini-chromosome. The DNA has an approximately 250-300 bp long origin abutted on either side by capsid protein gene on the left side and T-antigen gene on the right side of it. The genes slightly overlap the origin. The or5igin consist of two 72 bp long enhancer regions, next to it is six copies GC rich sequence and then it has 5 pentameric sequence and then it has AT rich imperfect palindrome sequence. The TATA region id found with in the pentameric sequences. This region acts as early promoter for the expression of T-Antigen gene, which on expression produces T-antigen, a 90KD protein. Occasionally the transcript by alternate splicing also generates small antigen-t.
~~~~ ----T----ß-Ori-àVp1—Vp2—Vp3--- -~~~~
Early expression of T- antigen leads to replication of the viral DNA using the same sequences, which are used for early transcription. Early promoters and Late promoters are reasonably efficient.
By removing the capsid part of the DNA, it is possible to introduce any desired gene in its place. By retaining early functional gene this vector can be used for their episomal expression of a gene product s in a compatible cell line. By adding signal sequences it is possible to express gene products in secretory mode.
//------- Early-Pro—I----------X-gene------poly (A)-----//
Production of SV40-Derived Vectors;
David S. Strayer, Christine Mitchell, Dawn A. Maier and Carmen N. Nichols
Adapted from Gene Transfer: Delivery and Expression of DNA and RNA (ed.
Recombinant simian virus 40 (rSV40)-derived vectors are particularly useful for gene delivery to bone marrow progenitor cells and their differentiated derivatives, certain types of epithelial cells (e.g., hepatocytes), and central nervous system neurons and microglia. They integrate rapidly into cellular DNA to provide long-term gene expression in vitro and in vivo in both resting and dividing cells. Here we describe a protocol for production and purification of these vectors. These procedures require only packaging cells (e.g., COS-7) and circular vector genome DNA. Amplification involves repeated infection of packaging cells with vector produced by transfection. Cotransfection is not required in any step. Viruses are purified by centrifugation using discontinuous sucrose or cesium chloride (CsCl) gradients and resulting vectors are replication-incompetent and contain no detectable wild-type SV40 revertants. These approaches are simple, give reproducible results, and may be used to generate vectors that are deleted only for large T antigen (Tag), or for all SV40-coding sequences capable of carrying up to 5 kb of foreign DNA. These vectors are best applied to long-term expression of proteins normally encoded by mammalian cells or by viruses that infect mammalian cells, or of untranslated RNAs (e.g., RNA interference). The preparative approaches described facilitate application of these vectors and allow almost any laboratory to exploit their strengths for diverse gene delivery applications.
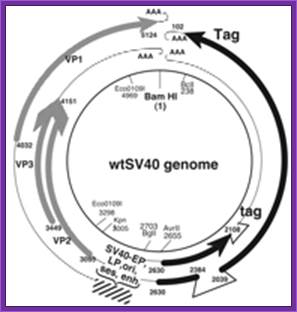
SV40-derived gene therapy Vectors. http://journals.prous.com/
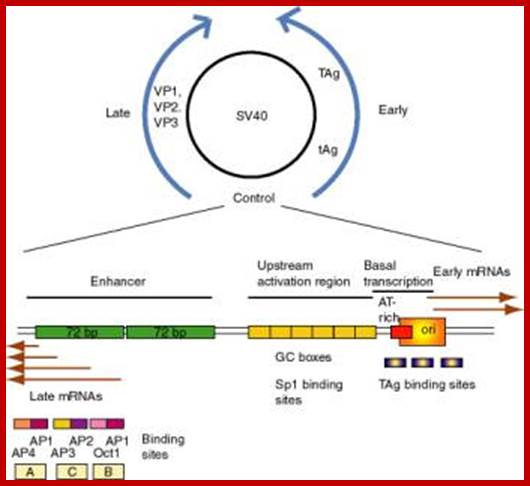
SV40 and its control regions
;http://www.bx.psu.edu/
Vaccinia derived vectors:
In 18th century, Edward Jenner used cowpox for immunizing human beings against cowpox disease. Since 1850 cow pox and other Vaccinia viruses are used as live vaccines. Even Fowl pox and Canary poxes are employed in developing vaccines. In fact, canary cowpox recombinant viral vaccines are in phase 1 and 2 trials.
Cow poxviruses and canary viruses can be used for developing vaccines.
Canary poxvirus is 250 x 300 nm size. The virus contains 100 or more proteins and it has lipid envelope with its own specific proteins. The genomic DNA enclosed is~180 to 200 kbp long, 67% is A/T rich and circularized by single stranded hairpin loops.
Viral particles exist in two forms one naked form (NV) and it is found in the cytoplasm, the second enveloped form that is enveloped with host cellular membrane with several glycoproteins (EEV).
Nearly 2/3 ds of the genome, central part of it, has replication functions and 1/3rd of the genome has no replication functions and this region is dispensable. One of the key enzymes produced by the viral genome is DNA dependent RNA polymerase, which recognizes unique promoter elements and initiate transcription.
The RNA-pol transcribes early class of genes, which are required for DNA replication. And few others are required for uncoating of the viral particle for replication.
At the onset of replication transcription of early genes stop and intermediate class of genes switch on. Among them three are transcriptional factors, which are required for late gene transcription. Late gene encodes structural proteins and early transcription factors and enzymes; all put together are packed into maturing viral particles. The fourth class of genes is expressed all the time, for they have early and late promoters upstream of the genes.
Vaccinia virus; http://en.wikipedia.org/
The Making of "the Unexpected Vector; www.bio.davidson.edu/Courses/Molbio/MolStudents/
As stated above, vaccinia undergoes homologous recombination during replication in infected cells. When used as an expression vector, this innate ability to recombine is used to introduce foreign DNA coupled to a vaccinia promoter, such as tk, into the viral genome (fig. 2). Numerous variants already exist, including those with indicators such as the lac-Z gene for blue-white selection (Cann). The steps below outline the construction of the vaccinia expression vector (fig. 2, by permission of Alan Cann) (Moss).
(1) Your favorite gene (YFG) is flanked with vaccinia DNA sequences, especially the vaccinia promoters and multicloning sites for cleavage and ligation. The following are often included:
- The promoters are necessary DNA sequences because the endogenous viral RNA polymerase binds here to initiate transcription (Unger). The promoter also determines the direction of translation for the insert, and more importantly the ability to express proteins (depending on how tightly regulated the promoter is).
- In addition, DNA sequences, such as the lacO/lacIq repressor system, that act in conjunction with promoters and also bind repressor molecules can regulate the induction of transcription (Unger). Hence, by adding or removing a particular substrate, expression of YFG can be turned on and off as necessary.
- Stabilizing elements such as transcription terminators can also be incorporated downstream of the multi-cloning site. These anti-termination elements signal the RNA polymerase to release the DNA template and stop transcription, and prevent pausing, pre-mature termination, and over reading which adversely affect plasmid replication (Unger).
- Finally, small open reading frames, known as ribosome binding sites, upstream of YFG, can be included to encourage binding and translation of the target sequence (Unger).
(2) The product (usually a plasmid with an ori and a marker gene) is then inserted into a cell infected with the whole virus. The whole virus must be used because it contains the necessary enzymes and factors within its core.
(3) Recombination during replication leads to insertion of YFG (i.e. the foreign DNA) into the viral progeny. The usual target of insertion is a nonessential region, so that virus retains its ability to replicate independently and the system can be maintained. The estimated incidence of successful insertion is approximately 0.1% (hey, I didn't say this was easy...). A major advantage of the Vaccinia vector is that at least 25,000 bp of DNA (a lot more than most vectors can handle) can be added to the Vaccinia genome without requiring any deletions.
(4) Controlling when and how much of YFG is expressed is easy because the poxvirus promoter sequences control the rate and time of expression, and you can regulate which promoters are in the system. The highest yields of protein are generally generated with the late promoters.
(5) Virus plaques can finally be screened by DNA hybridization or for expression of your favorite protein.
With the rapid discovery of new genes, especially from the Human Genome Project, comes the daunting task of understanding how the products of these genes are synthesized, regulated, and used within cells. Vaccinia virus, as a vector for expression systems, is a powerful addition to the range of molecular methods available for such purposes. The use of Vaccinia allows temporal, as well as quantitative regulation of protein expression. More importantly, Vaccinia is large enough to accommodate several gene inserts while preserving the entire length of its DNA. Finally, as an infectious agent, it can target specific cells for insertion, and may thus be employed in gene and cancer therapy. Led by Vaccinia, the Poxviridae may no longer be considered the scourge of the world, but rather powerful tools for advancing research and therapeutic avenues.
Various transfer vectors have been created using different kinds of promoters such as TK, 7.5K, 11K, CPK. T7-10 etc. Among them late gene promoters are very strong and efficient promoters, ex. 11K.
By recombinant methods T7 promoter has been introduced into the Vaccinia genome and it is the most expressive promoter. However to express it, one has to introduce T7 RNA pol gene in expression mode. Any foreign gene can be constructed under T7 promoter. In fact any of the foreign genes can be inserted into any of the 55 genes, but widely used one is Thymidine Kinase gene for in vitro manipulation.
First construct a plasmid with required composition of genes and a segment of DNA for homologous recombination with wild type virion DNA. The TK-L and TK-R are the left and right end borders of Thymidine kinase gene from wild type
----ColE1-ORI—TK-L<—bGal—ßK11-P---P-7.5--->-Gene-X-àTK-R---~= Circular ends.
Infect cells with the plasmid construct and wild type viral DNA and allow recombination, which can be screened for the recombinants easily. When grown in the presence of deoxy 5’ Bromo uracil, the wild type thymidine kinase undergoes mutation and it does not function, but recombinant has only the borders and not the entire TK gene, so the recombinants survive and wild type die. Glycover can be used to screen for the absence of Thymidine kinase.
Another way to identification, whether or not recombination has taken place or not, is add b-Galactoside for the b-Galactosidase as the marker gene is expressed in the recombinant, and the color generated indicated the construct is working as the recombinant.
Cell lines used in these protocols are Monkey kidney cells called CV-1 and Rabbit kidney cell called RK-13.
The virus has a wide host range. Such antigen expressing recombinant viral DNA can be used as live vaccines.
Replication of the viral DNA takes place in the cytoplasm and not in the nucleus. The viral derived vectors have been used to develop live recombinant viruses. These viruses are also used for developing vaccines against Rabies G protein, Hepatitis B surface antigen, Influenza NP and HA proteins, VSV-N and G proteins, HSV glycoproteins-type-1. The same were used in clinical trials and found that the animals immunized show immunity against specific infection.
Vaccinia has an advantage in cloning a large sized DNA containing different structural regions of different infectious agents, so as to express as single proteins so as to obtain immunity against multiple infections!
Adeno Viral derived vectors:
Adenovirus, though an animal virus, now found to infect human beings. It is now a reality that quite a number of animal viruses by mutation are infecting human beings. A good example is Corona virus, the DNA has undergone certain deletion and changes in nucleotide sequence, and as a consequence this virus has become a potential killer.
The virus has wide range of hosts and causes oesophyrengeal infections, not very serious. The virus is icosahedra in shape, 130nm, and nonenveloped. The DNA is doubling stranded with sticky ends and 36 to 38 kbp long; and surprisingly linear, but the binding of 50 KD proteins protects the ends at 5’ region at either end. The genome has 133bp long terminal repeats. It has a total of 100 map units.
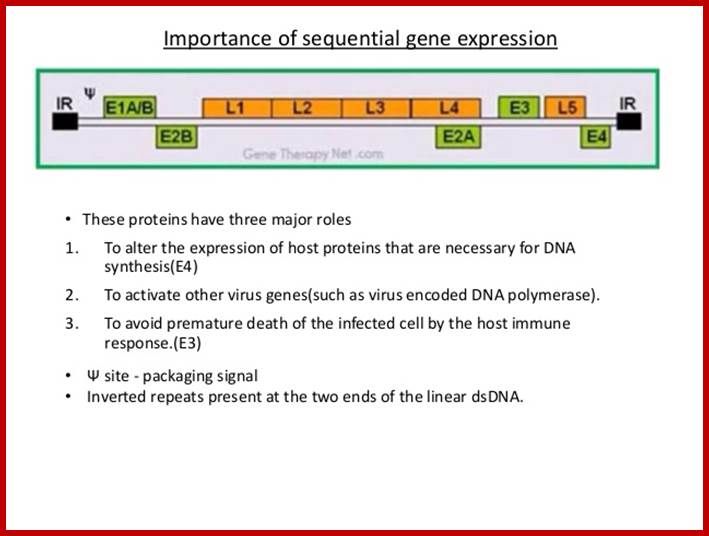
Early genes expressed are E1, E2, E3 and E4. Early gene-1 is a transcription factor, which activates several host cell gene expression. They also activate viral gene expression and help the viral DNA replicative functions. The late genes code for capsid proteins. During lytic phase the viral DNA is replicates to several thousand copies and using late gene products they produce ADV particles and lyse the cells.
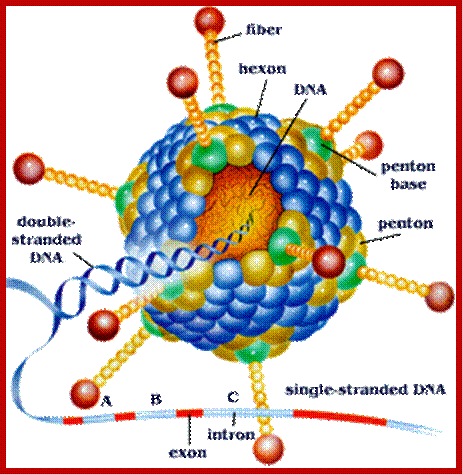
Structure of Adenovirus; https://microbewiki.kenyon.edu
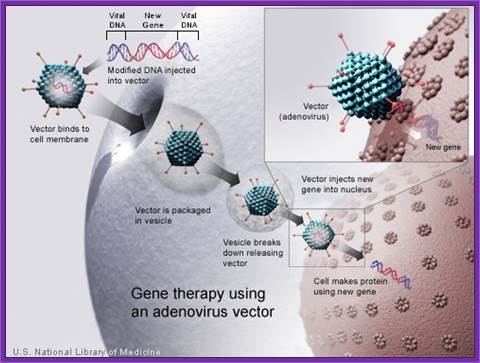
Adeno viral life cycle; infection; this infection cycle of ADV used for Cancer therapy; https://microbewiki.kenyon.edu
The left end of the genome, nearly 12% has replicative functions. This can be cloned into cell lines as integrated part. This segment of DNA in human kidney helper cell lines provides functions for defective recombinant virus.
Early promoters used are E1a and E1b. An early gene can be replaced with a foreign gene of interest can be cloned under the promoter of E1a. Transfection of such DNA into helper cell lines like 293 produce packed ADV particles with recombinant DNA. Such viruses can produced in large amounts, scaling up of the production of recombinant viruses is must for application. Such viruses can be used as nasal sprays in combating certain diseases. Example Cystic fibrosis is due to the defect in producing a channel protein for the transport of chloride ions. Recombinant viruses containing cystic fibrosis gene has been cloned and such ADV’s are used as nasal sprays and found curative effects. The problem is human body develops immunity against ADV’s, and then the problem remains the same.
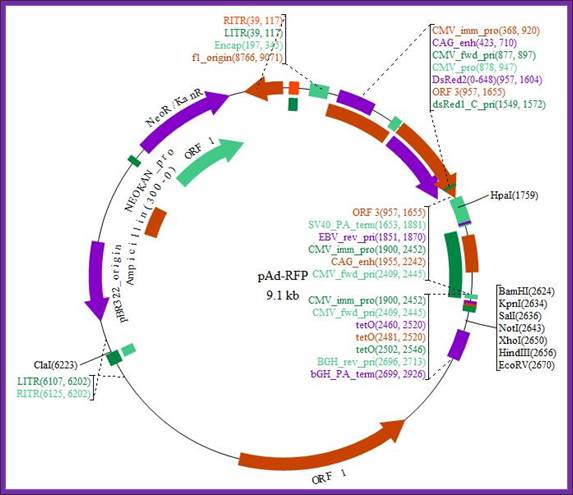
Plasmid map of pAd-RFP; http://www.biovisualtech.com/bvplasmid
pAd-RFP
Gene/insert name: RFP
Insert size (bp): Unknown
Species of gene(s): Other
Vector backbone: pAdTrace-TO4 (Search Vector Database)
Type of vector: Mammalian expression
Backbone size (bp): 9075
5' Sequencing primer: na (List of Sequencing Primers)
Bacteria resistance: Kanamycin
High or low copy: High Copy
Grow in standard E. coli @ 37C: Yes
Selectable markers: Neomycin
If you did not originally clone this gene, from whom and where did you receive
the plasmid used to derive this plasmid: Roger Tsien, UCSD
Plasmid Provided In: DH5a
Principal Investigator: Tong-Chuan He
Comments: This is an RFP expressing adenoviral vector compatible with the
AdEasy system.
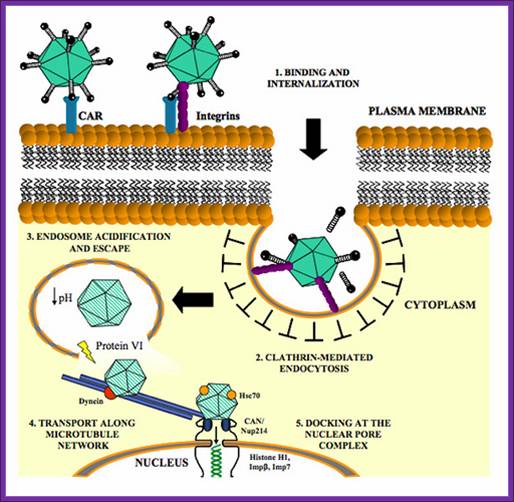
Targeted Gene Delivery Using Modified Adenoviruses; http://www.bing.com
Packaging cell lines
From An efficient method of directly cloning chimpanzee adenovirus as a vaccine vector, Dongming Zhou1, 2 Xiangyang Zhou1, 2 Ang Bian1 Hua Li1 Heng Chen1 Juliana C Small1 Yan Li1 Wynetta Giles-Davis1 Zhiquan Xiang1 and Hildegund C J

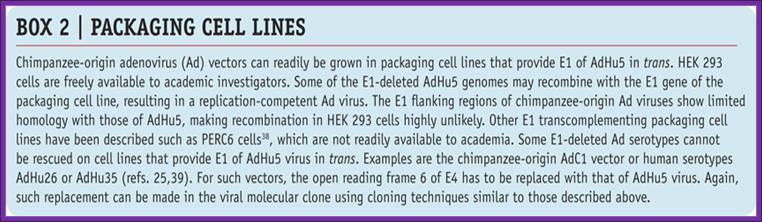
First generation of Recombinant Vector:
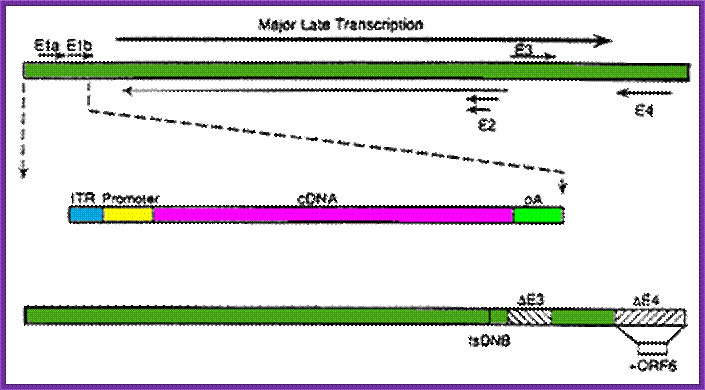
Adenoviruses can be made into replication-defective vectors by removing the E1 region located in the left side of the viral DNA, this early transcript encodes for proteins that activate the transcription of all other viral proteins. Typically, an expression cassette encoding the therapeutic gene is inserted into the deleted E1 region and the resultant recombinant viral vector is propagated in a cell line that expresses the adenoviral E1 genes (see diagram two).
 Adenovirus
as an animal vector; http://www.slideshare.net/
Adenovirus
as an animal vector; http://www.slideshare.net/
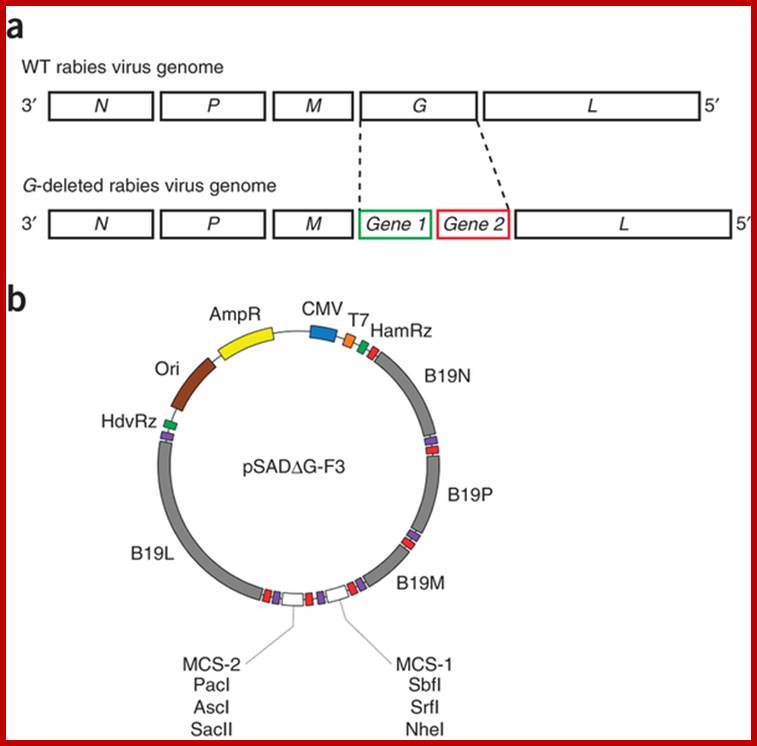
Design and generation of recombinant rabies virus vectors; Fumitaka Osakada
& Edward M Callaway; http://www.nature.com/
Second generation
To further limit the leaky expression of viral proteins and expand the capacity of adenoviral vectors, further deletions have been made in the adenoviral genome. Viruses with additional deletions in the E2 or E4 regions have been made by a number of researchers, creating recombinant vectors that have to be propagated in special complementing cell lines, expressing the E2 or E4 regions, e.g. 293-C7 (E2), 293-ORF6 (E4) or 911-E4.
Third generation
The entire adenoviral genome, with exception of the essential cis elements (5’ and 3’ ITRs and packaging signal) can be removed to generate recombinant gutted (or gutless) adenoviral vectors. . They also have a large capacity for exogenous DNA, being able to package up to 36 kb of transgene. However, their production is a tricky process, given that they can only be propagated in the presence of a helper adenovirus.,
The phenomenon of site-specific recombination can be effectively employed in order to reduce the contaminating levels of helper virus to less than 0.1%. In this approach the packaging signal of the helper virus is flanked by two recombination signals (e.g. loxP sites). The virus is then propagated in a special cell line that expresses the recombination protein that recognises these signals (e.g. CRE recombinase). The packaging signal is thereby excised from the helper virus and its genome can no longer be packaged into the capsid proteins, thus allowing for efficient packaging and production of the vector genome only. The gutted adenoviral vectors perhaps represent the best hope for an effective, large capacity vector based on the adenoviruses, however, until the efficiency of vector production is significantly improved, it is unlikely that these vectors will be widely applied in the clinic.
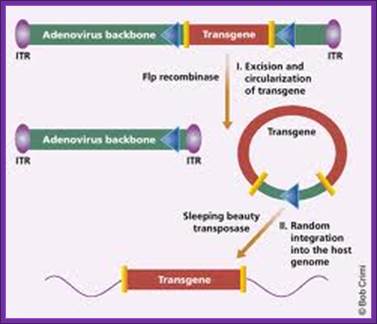
ADV vector
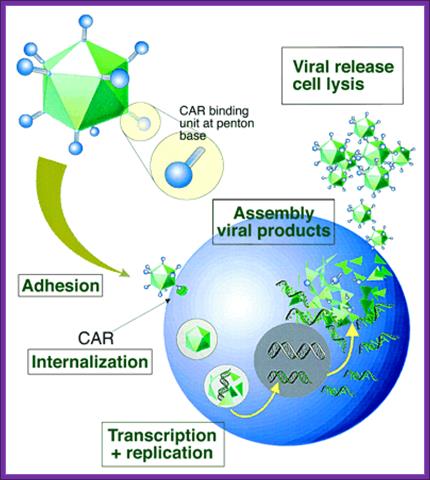
ADV Viral life cycle
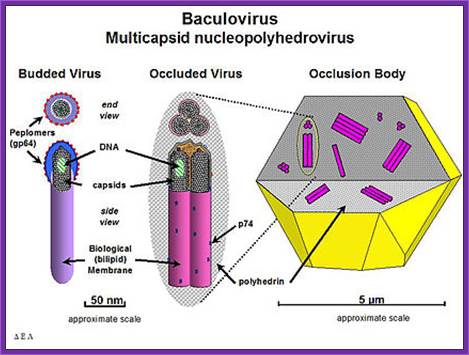
Baculo viral vectors:
Baculoviruses are a diverse group of insect viruses. Autograpaha californica is a multinuclear polyhedral virus (ACMNPV), and Bombax mori polyhedron viruses (BMNPV) are just two such examples.
Genome size of these viruses is about 128 to 200 kbp (ACMNPV =128 KBP) AND IN CIRCULAR MODE. Insect cell lines used for the expression of viral genomes are Spodofora fugiperda (Sf-9 cell lines). On infection viruses multiply within epithelial cells of gut.
Viral genes are expressed in temporal fashion, such as early, late and very late.
Early gene products initiate its DNA replication and also activate the expression of late genes. This results in the multiplication of the genome as well as viruses, which in turn are budded off as enveloped viruses (Env), which further infect new set of cells.
Expression of very late genes is about 18 to 20 hrs after infection, which code for polyhydrin proteins. At this stage the host cell nuclear membrane proliferates and primary viruses are occluded among the polyhydrin matrix proteins (29KD).
In order to use them for expression foreign genes first clone polyhydrin gene along with flanking regions into a plasmid for recombination purposes.
https://en.wikipedia.org
Invented by Dr. Frederick M. Boyce, BacMam is a baculovirus-mediated gene transfer technique that has gained widespread use because of advantages when compared to other transfection methods, (for reviews see, Kost, T.A. et al, In addition, BacMam has been found to have inherent flexibility over stable cell lines, which has contributed to its adoption as a standard gene transfer technique (wikipwdia).
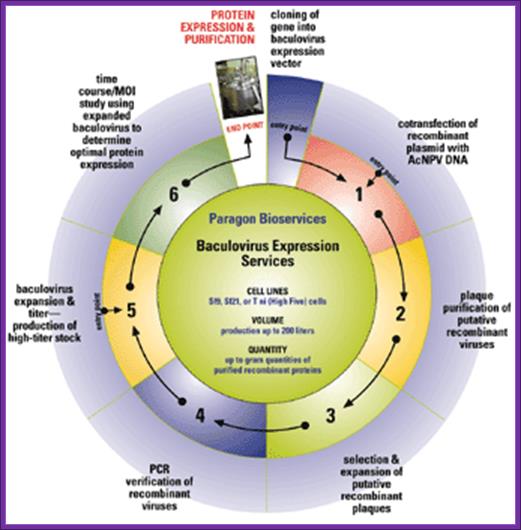
Starting from a DNA sequence, cDNA or recombinant virus; Cloning into commercially available expression vectors (± affinity tags). Veracity of construct demonstrated by restriction digest analysis and sequencing; Co-transfection with AcNPV DNA; Plaque purification of recombinant viruses; Expansion and PCR verification of recombinant viruses; Baculovirus expansion and titer - production of 1L high-titer stock; Time course and MOI study to optimize protein expression; http://www.paragonbioservices.com/
~~~--Col.E1ori—I-V-DNA-I----polyhydrin----I-V-DNA-I---Amp^+--~~~
~~~ = End of a circular plasmid.
V-DNA = Viral DNA as flanking regions.
Then delete the polyhydrin gene and in its place put a desired gene under a proper polyhydrin promoter. Then co-infect insect lines with plasmid DNA and the viral DNA. In insect cells, homologous recombination results in the incorporation of the desired gene. The viral DNA replicates to 800-1000 copies per cell and later generates primary virions, which can further infect.
Mouse IgG against Pseudomonas aeruginosa lipoprotein was expressed in Baculo viral recombinants. In this case Transfer vector was constructed in such a way both light and heavy chain genes are cloned separately under specific polyhydrin promoters with flanking DNA from the virus. When wild type viral DNA and the transfer DNA are co infected into insect cell, homologous recombination results in the integration of both IgG genes under promoters which are expressed in insect cells. In order to scale up the production one can directly infect larvae instead if cells.
For example recombinant Trichoplusia larvae containing human Adenosine deaminase gene, found the expressed protein level was found to be 3-5% of the cellular proteins. For continuous production the desired gene has to be to be constructed under the early gene promoter for they don’t require any viral gene products for expression. It is also possible to integrate the gene construct into host cells and express continuously to get more of the recombinant protein. Advantage of using Baculo viral viruses is one can clone a DNA fragme4nt of the size of 15-20 kbp long. Direct injecting modified virus can achieve infection and growth. Proteins expressed in insect are found to have all characteristic post-translational modification, but in some case glycosylation is same as that of mammalian system.
pAC UW31 transfer Vector:
Derived from Baculo viral genome.
~~-M13 ori—Amp^+->---ColE1 ori----ACNPV 2099—SV40P-X—<-EcoR1,
BglII, Xba1--Phy/P-BamH1—XàPoly(A)—ACNPV 2493bp----~~
~~ = Circular end of the transfer vector.
X = foreign genes.
Two gene can be expressed understand protein-protein interaction.
Bac pAK transfer vector:
~~---<Bsu-361---i-P/PolyH-Lac-Z-à I I-à-ßBSU361-ORF1629-Ori-Amp^+~~
ORF is required for viral replication.
Promoter Polyhydrin drives Lac-Z for color for non-recombinant plaques.
pBac pAK 8 and 9: transfer vectors:
This vector is designed for high level of expression. It consists of strong polyhydrin vector flanked by homologous sequences for recombination. The MCS has 18 sites and the other features like M13 origin, Amp^+ and ColE1 Ori.
~~--M13ori—Amp^+à--pUC ori----V-DNA—P-PH-MCS-poly (A) V-DNA--~~~
~~ = Circular ends.
V-DNA = flanking viral DNA.
McsinPAK8=Bamh1.Ssc1.Pst1.stu1.Xho1.Bst-B.Xba1.BglII.Asp71811.Sma1.Eag1.Not1.Pac1. Pac1.
In PAK the mcs are in reverse.
Retroviral derived vectors:
There are a large number f RNA viruses, among them retroviruses are important, which are also called retroviruses. When they infect compatible cells, then virus replicates and integrates into the host genome as a cDNA but its size is little longer than the original size of the viral RNA. It is only after activation of cells, which have the integrated viral genome, produce viral particles.
When the viral genomic RNA replicates it produces ds cDNA with long terminal repeats (LTR) at their respective ends, hence the size of this is slightly larger than the viral genome.
Such ds viral DNA can be retrieved. The LTR segments and viral packaging sequences can be used to construct an expression vector. The LTR sequences also contain promoter elements U3 R U5-Ψ-. In between the LTR segments one can introduce required genes under specific promoters. And also one can have a selection marker gene under specific promoter. If the vector has viral packaging sequences the viral DNA can be packaged into a viral particle and obtain them in large numbers and the same can be used for infecting a particular tissue. Upon infection the viral particle release the DNA into cytoplasm and the circular DNA enters into the Nucleus, where using LTR sequences the DNA integrates into the host genome. The transfected cells can be screened with a selection marker gene and the cells can be amplified and the same can be used to obtain the gene product or the transgenic cells can be used for genes therapy.
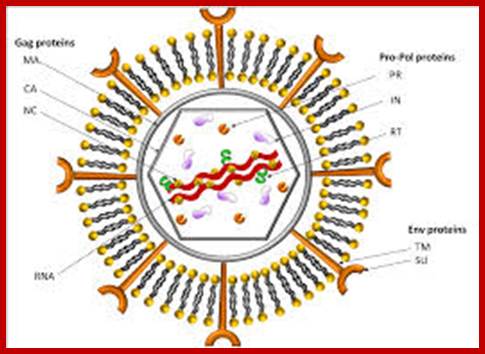
www.intechopen.com
How to develop a live recombinant virus:
In order to develop a live recombinant virus first one has to transfer viral capsid genes into specific host cells, where the genes are inserted in expression mode but regulatable and inducible?. Such cells can be maintained and expanded; such cells are called helper cell lines.
Such cells are transfected with above-mentioned viral construct. Then the cells are stimulated to produce capsid proteins. As the vector DNA has sequences for packaging, the capsid proteins bind to such sequences and complete packaging leads to full viral particle production. If such cell line also have specific envelop protein genes, then the envelope proteins that incorporate into the viral envelop, which can targeted to specific cell type.
When the tissue is infected with the recombinant viruses, the released DNA goes into the nucleus where it gets integrated into the chromosomal DNA using LTR sequences and rest of the DNA gets degraded.
Application of this method is very important for one can deliver the viruses into specific tissues, without causing any adverse effects. Any constructs with LTR sequences can also used directly transfer the construct into the cells by direct transfer by any one of the Transfection methods. The transfected DNA gets integrated into the host genome using LTR sequences. It is greatly facilitated if the cells are mitotically active. This method has been employed in gene therapy.
-U3-R-U5-I~~-P---X-I-I-gene—Ttr—I---P—Kan+ --Ttr—U3-R-U5-
U3RU5 = LTR sequences,
~~ = Packaging sequences
II = Introns.
Ttr = transcriptional terminator.
Design of Retroviral Vectors and Helper Cells for Gene Therapy:
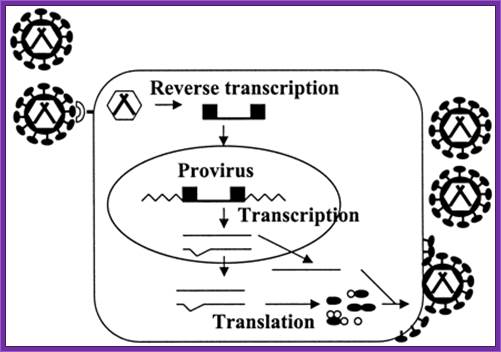
Design of Retroviral Vectors and Helper Cells for Gene Therapy; Retrovirus enters thoughthe binding to a cell receptor, with the release of the its genome it gets reverse transcribed, then the viral DNA gets integrated into host genome. Cellhas machinery to transcribe the integrated viral genome and the rnaS ARE PROCESSED AND TRANSLATED; WHICH GETS ASSEMBLED INTO AVIRAL PARTICLE AND THE SAME IS RELEASED BY BUDDING. http://pharmrev.aspetjournals.org/
Replication cycle of retroviruses: A retrovirus binds to a receptor on the cell surface (shown as a crescent), enters the cell, and reverse transcribes the RNA into double-stranded DNA (shown as a line flanked by black boxes), viral DNA integrates into the cell chromosome (shown as zigzag lines) to form a provirus. Cellular machinery transcribes and processes the RNA (shown as thin lines), and translates the viral proteins (shown as black ellipses and white circles). Viral RNA and proteins assemble to form new viruses, which are released from the cell by budding.
Design of retroviral vectors and helper cells for Gene therapy
;http://pharmrev.aspetjournals.org/content
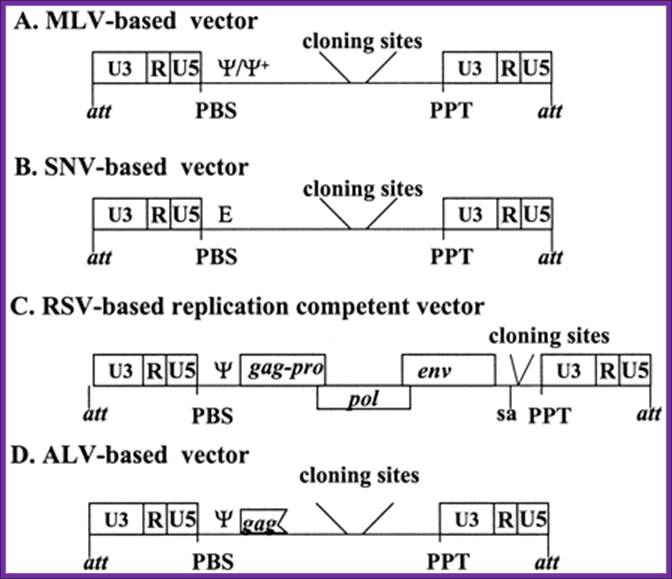
General structures of the oncovirus-derived vectors: All vectors are shown in DNA form. A, MLV-based vector; B, SNV-based vector; C, RSV-based vector; D, ALV-based vectors. Truncated gag contains a part of the packaging signal.
LinX retroviral expression system- for packaging shRNA expression vectors.
Greg Hannon at CSHL:
The LinX retroviral expression system created by is an efficient
method for retroviral packaging. It produces amphotropic retrovirus that infects
cells from a broad range of mammalian species. This line is derived from human
embryonic kidney (HEK 293) cell line. The viral gag, pol and env genes-
necessary for particle formation and replication- are stably integrated into
the genome of the LinX packaging cell line. The separate introduction and
integration of the structural genes minimizes the chances of producing
replication-competent virus due to recombination events during cell
proliferation2,3. The retroviral expression vector provides the viral
packaging signal (Ψ), the shRNA to the target gene and a puromycin
resistance marker. Transfection of the pSM2 self-inactivating retroviral vector
into the LinX packaging cell line produces replication-incompetent virus that
can efficiently transfer genes into a variety of mammalian cell types.
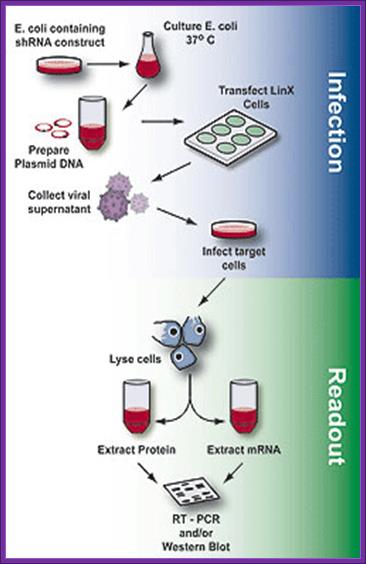
Overview of the process of generating packaged virus particles using shRNA and LinX cells, infection of target cells and assaying for mRNA or protein expression (readout). http://www.bioxys.com/
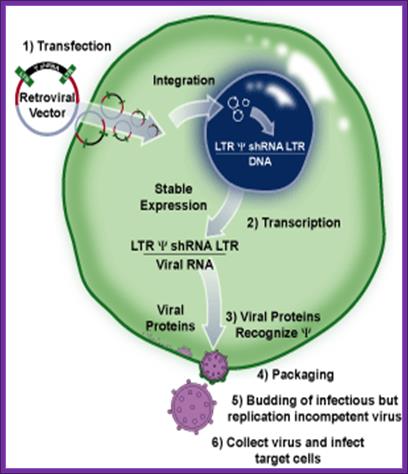
http://www.bioxys.com/
Once the packaging cell line is transfected with a retroviral expression vector that contains a packaging signal (1), the viral genomic transcript containing the target gene and selectable marker are packaged into infectious virus within 48–72 hrs (2-4). Virus produced in this way can infect target cells and transmit target genes; however, it cannot replicate within target cells because the viral structural genes are absent.
The psi-2 Packaging Line of Mann, Mulligan and Baltimore:
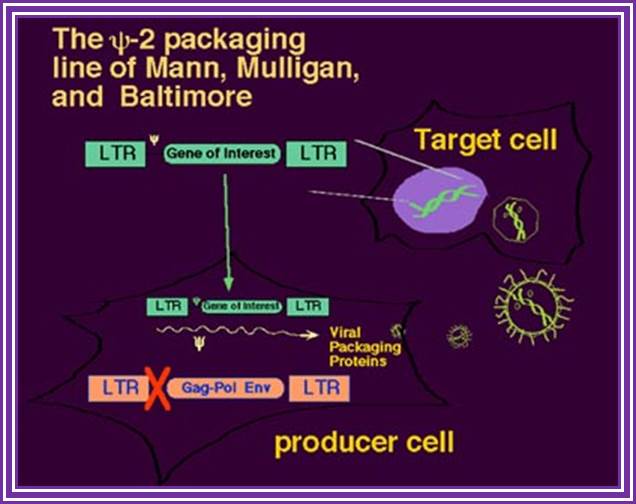
The use of recombinant retroviruses was pioneered by Richard Mulligan and David Baltimore with the Psi-2 lines and analogous retrovirus packaging systems, based on NIH 3T3 cells (10, 11). Such helper-defective packaging lines are capable of producing all the necessary trans proteins--gag, pol, and env-- that are required for packaging, processing, reverse transcription, and integration of recombinant genomes. Those RNA molecules that have in cis the Psi packaging signal are packaged into maturing virions.
Cell Biolabs Inc. Retroviral Expression and Packaging:
· Platinum (Plat) cell lines provide a powerful, stable method for producing high levels of retroviral structure proteins. Plat-E and Plat-A cells stably express the gag, pol, and env genes, allowing retroviral packaging with a single plasmid transfection to produce recombinant ecotropic and amphotropic virus, respectively. Pantropic retrovirus may also be produced by transfecting Plat-GP cells with the expression vector and a second vector containing VSV-G.
· 293RTV cell line is a permanent cell line established from the parental 293 cell line and selected for high-yield retroviral production. This cell line stably expresses the SV40 large T antigen, and will package retrovirus upon transfection with gag, pol, and env vectors plus an expression vector containing your gene of interest. These packaging vectors are available individually.
Our retroviral expression vectors are available with a variety of backbones for general gene expression purposes or specific transduction of stem cells. For your convenience, Platinum Retroviral Expression Systems contain both a Platinum packaging cell line and the vectors required for efficient packaging of high-titer retrovirus.
Few Reporter Genes Used for Animal transformation:
B-Galactosidase: (116KD), substrates- X-Gal produces purplish color, Blue Gal produces deep blue color, Galacton –emits luminescent light.
B-D Glucuronide dehydrase: substrates- (MUG), 4methyl umbilliferyl b-D Glucuronide, produces fluorescence.
B-Glucorunidase: substrate-5-Bromo4-chloro3-indolyl-b-D Glucuronide- produces fluorescent product.
Chloramphenicol acetyl Transferase: substrate Chloramphenicol.
Luciferase: Luciferin + ATP- generate luminescence.
GFP (Green Fluorescence protein- (27KD): Produces green light when exposed to UV or blue light. GFP gene is isolated from Jamaican chick beetle (Kitty boo called Pyrophorene plagiophthalamus). This jellyfish has two organs one at abdominal region and the other on its head. It has four genes, which produce different colors such as green, yellow green, yellow and orange.
There are a large number of vectors for expression of genes in animal tissues. Many vectors have been specially created for targeting their products to certain destination. This is possible if target specific signal sequences are ligated in frame with a designated gene, the protein produced goes through the processing and ends in that specific target.
Some promoters used for expression in Animal cells:
- SV 40 early gene promoter with enhancer.
- Mouse mammary tumor viral promoter with Dioxane response elements.
- b-Actin gene promoter.
- Metallothionin promoter with NRE or GRE elements.
Some Promoters used in some animals:
- Chick; ALV Avian leukemia viral promoter.
- Cow: BPV- Bovine Papiloma viral promoter.
- Fish: Metallothionin promoter. Human, Bovine growth hormone genes.
- Pig: MM tr- Mouse mammary tumor viral promoter. HGH gene.
Murine leukekemia viral promoter- gene cloned rat growth hormone.
- Rabbit: MM tr- HGH.Sheep: MM tr- Human GH, TK, bovine GH; ovine b-lacto globulin promoter for human factor-IX and gene XI anti Trypsin factor.
- Goat: Prolactin promoter- Tissue specific plasminogen activating factor.
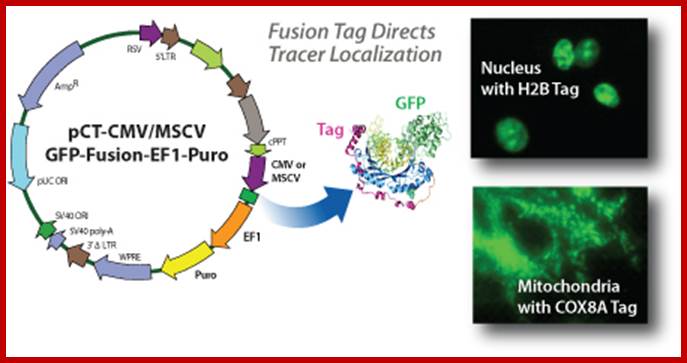 Compartment Tracing using Lentiviral
Fluorescent Protein Reporter Vectors; http://www.biocat.com/
Compartment Tracing using Lentiviral
Fluorescent Protein Reporter Vectors; http://www.biocat.com/
Cytomegalovirus expression vector
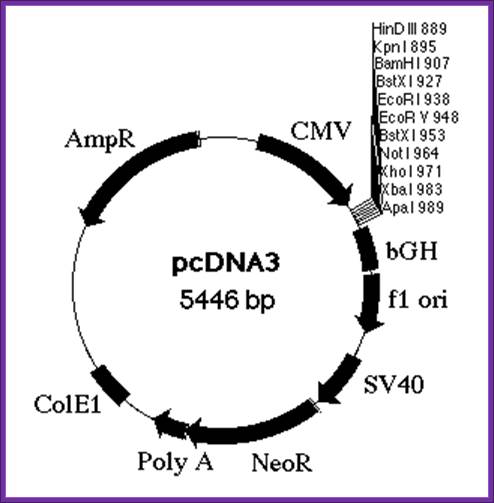
CMV vector also contains SV40 promoter
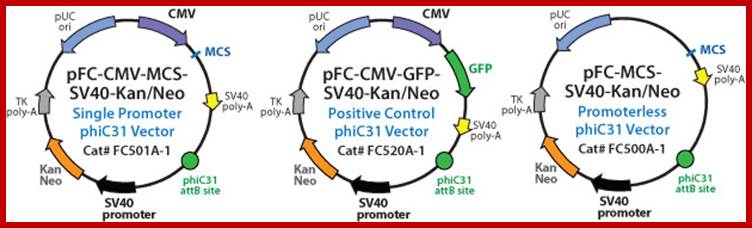
http://www.biocat.com/genomics/